Chemistry: Measurement, Significant Figures, and Problem Solving
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
65 Terms
Scientific Notation
A way of writing very large or very small numbers using powers of 10.
Accuracy
How close a measurement is to the true value.
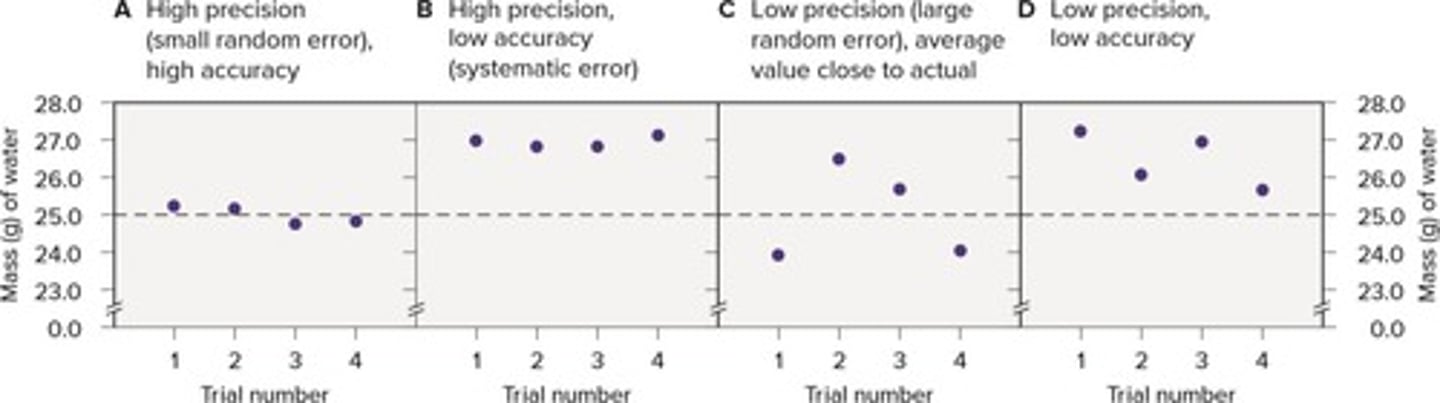
Precision
How close repeated measurements are to each other.
Significant Figures
They reflect how 'certain' (precision) we are in the measurement value and help us know how 'correct' (accuracy) that value is.
Exact Number
A number with no uncertainty in its value; it is known with 100% certainty.
Non-Exact Numbers
Quantities derived from measurements other than counting that have a certain level of uncertainty.
Measurement Uncertainty
The level of uncertainty associated with measurements derived from practical limitations.
Expanded Notation
The form of writing numbers that shows the value of each digit.
Coefficient
The number 'a' in scientific notation, where 1 ≤ a < 10.
Exponent
The number 'n' in scientific notation that indicates the power of 10.
Rounding Rules
The proper methods to adjust numbers to reflect significant figures.
Example of Scientific Notation
4.5×10^4 for large numbers and 0.000000451 for small numbers.
Adjusting the Exponent
To increase the exponent by 1, decrease the coefficient by a factor of 10.
Counting Measurement
An example of an exact number, such as the number of people in a room.
Defined Quantities
Relationships defined by exact quantities, such as 1 ft is exactly 12 in.
Measurement Process Limitations
The practical limitations that lead to uncertainty in non-exact numbers.
Power of 10
The format used in scientific notation to indicate the scale of the number.
Decimal Movement
The process of moving the decimal point to adjust the coefficient in scientific notation.
True Value
The actual value that a measurement aims to reflect.
Finely Measure
The ability to measure with a high degree of precision.
Mass Example
A balance reads a mass of 12.345 g, indicating a specific level of precision.
Estimated Digit
The last digit (rightmost) recorded for a measurement, which is always estimated.
Mass Measurements
All digits displayed are considered significant figures.
Volume Measurements
Use the bottom (or top if inverted) of the meniscus to estimate.

Meniscus
Curved top surface of a liquid.
Non-zeros
These are always counted as significant in reported measurements.
Zeros at the Trailing End
These are significant only if a decimal is explicitly written.
Captive Zeros
Zeros that are 'captive' between non-zeros are significant.
Example of Trailing Zeros
90.0 g is significant, while 90 g is not.
Example of Captive Zeros
105 mL is significant, while 0.015 L is not.
Counting Significant Figures
Use a set of rules to determine how many significant figures are in a number.
Significant Figures in 0.005090 mg
This measurement has four (4) reported significant figures.
Reporting Significant Figures in Calculations
The calculated number cannot contain more certainty than the measurement itself.
Adding Zeros
Must add zeros (and sometimes also use scientific notation) if more digits need to be included.
Multiplication/Division Rule
The calculated value has its sig figs limited by the number from the calculation with the fewest number of sig figs.
Addition/Subtraction Rule
The calculated value has its sig figs limited by the number from the calculation with the least precise place value.
Example of Reporting with Four Sig Figs
Report 85,327 g with four total sig figs.
Example of Including More Digits
Report 85 mg with four total sig figs.
Non-zero digits
Always significant.
Trailing zeros
Significant if they are at the end of a number and a decimal is explicitly written.
Leading zeros
Not significant as they are not at the trailing end nor captive between non-zeros.
Reported significant figures
The number of significant figures in a measurement, e.g., 0.005090 mg has four significant figures.
Calculations with significant figures
The calculated number cannot contain more certainty than the measurement itself.
Example of truncation
Report 85,327 g with four total significant figures.
Example of multiplication/division
For 15.227 nm × 1.054 nm, the result is limited by the number with the fewest significant figures.
Example of addition/subtraction
For 3.45 g + 1.1 g, the result is limited by the least precise place value.
Next lecture preparation
Read Sections 1.6 in the text covering mathematical treatment of units, unit conversions, and using scientific formulas.
Dimensional analysis
The general process of treating units as mathematical quantities in a calculation.
Factor-Label Method
A method that utilizes ratios of equivalent units called conversion factors for unit conversions.
Conversion factor
A ratio between equivalent measurements each expressed in different units.
Scientific formula
A mathematical equation that expresses a relationship between different properties.
Density formula
The formula for density, 𝑑, is a ratio between its mass, 𝑚, and volume, 𝑉: 𝑑=𝑚/𝑉.
Unit conversions
The process of converting a quantity from one unit to another using conversion factors.
Example of unit conversion
1 ft = 12 in (equivalent measurements).
Example of scientific formula
The density of a metal block measuring 2.50 cm x 4.30 cm x 1.90 cm with a density of 7.34 g/cm3.
Combining unit conversions with scientific formulas
Example: What is the density (in g/cm3) of a metal block that has a mass of 0.255 kg and measures 34 mm x 46 mm x 56 mm?
Mathematical Treatment of Measurement Results
Using mathematical equations to describe the relationships between properties.
Example of dimensional analysis
Acts like an 'insurance policy' - if the final units match the property of the unknown, it's a strong indication it was done correctly.
Blueprint for unit conversions
Given unit × desired unit / given unit = desired unit.
Example of radius conversion
The radius of a single neon atom is 1.60 Å. What is its radius in meters? 1 Å= 10^-10 m.
Chemical problem-solving strategies
Various approaches to tackle chemical problems effectively.
Learning Objectives
Use dimensional analysis, factor label method, and scientific formulas to calculate properties.
Phases and Classification of Matter
Different states in which matter exists, categorized based on physical properties.
Physical and Chemical Properties
Characteristics that define the behavior of substances in physical and chemical processes.
Measurements
Quantitative assessments of physical quantities.