Chemical Interactions in Biology: Bonds, Polarity, and Salt Bridges
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Chemical interactions
Interactions that dictate how biology occurs.
Chemical Bonds
Interactions between atoms that keep them close together.
Covalent Bond
Interaction where two atoms share valence electron(s).
Ionic Bond
Bond between positively and negatively charged atoms (full charges on atoms from electron exchange allow bonding).
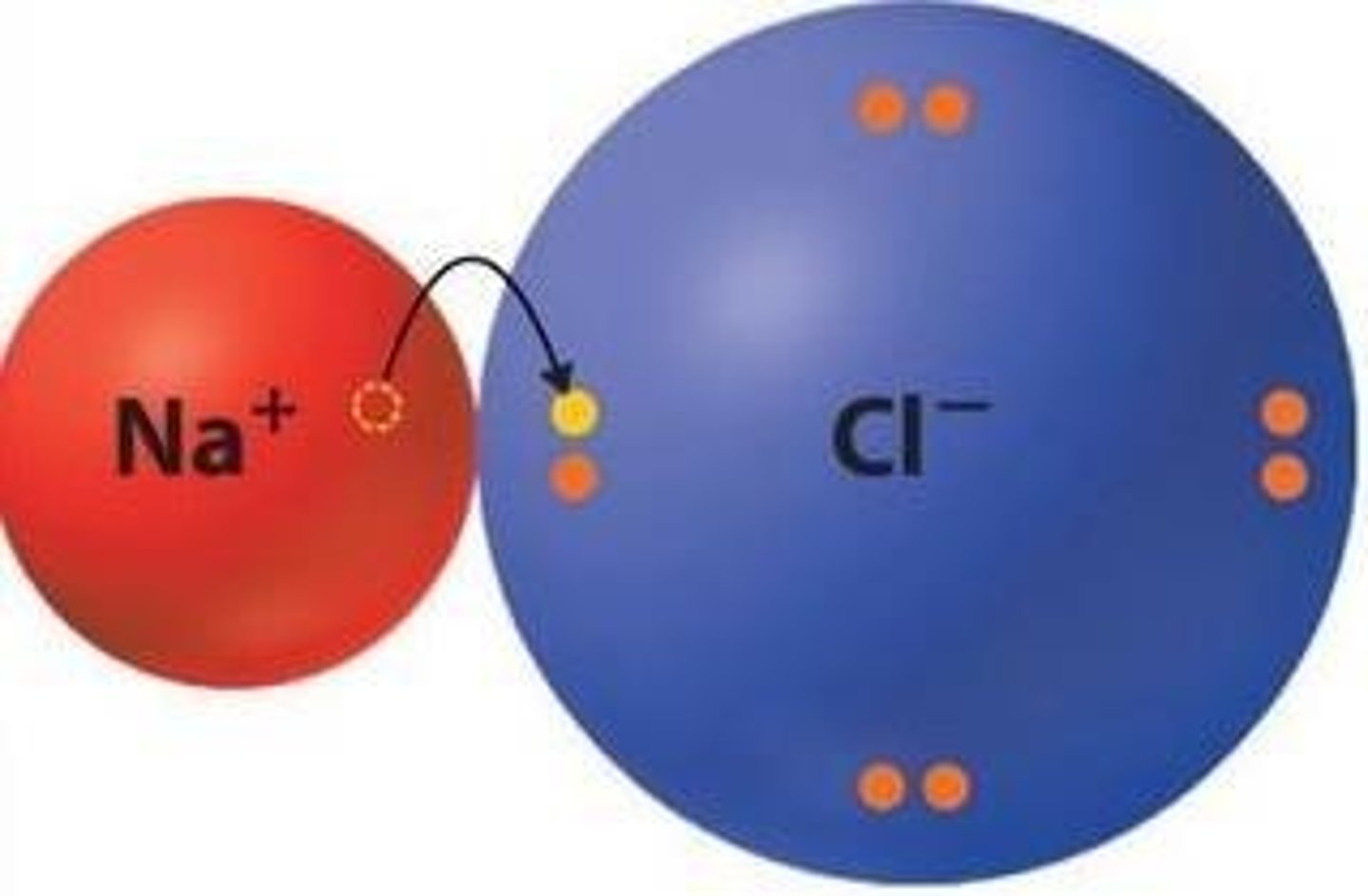
Polarity
Unequal sharing of electrons in a chemical bond, resulting in partial charges on the involved atoms.
Electronegativity
Tendency for an atom to attract electrons towards itself (over other atoms in a chemical bond).
Dipoles
Chemical bonds with positively and negatively charged parts (atoms).
Ion-Dipole Interactions
Ionic charges (cations and anions) and opposite partial charges (dipoles) interact.
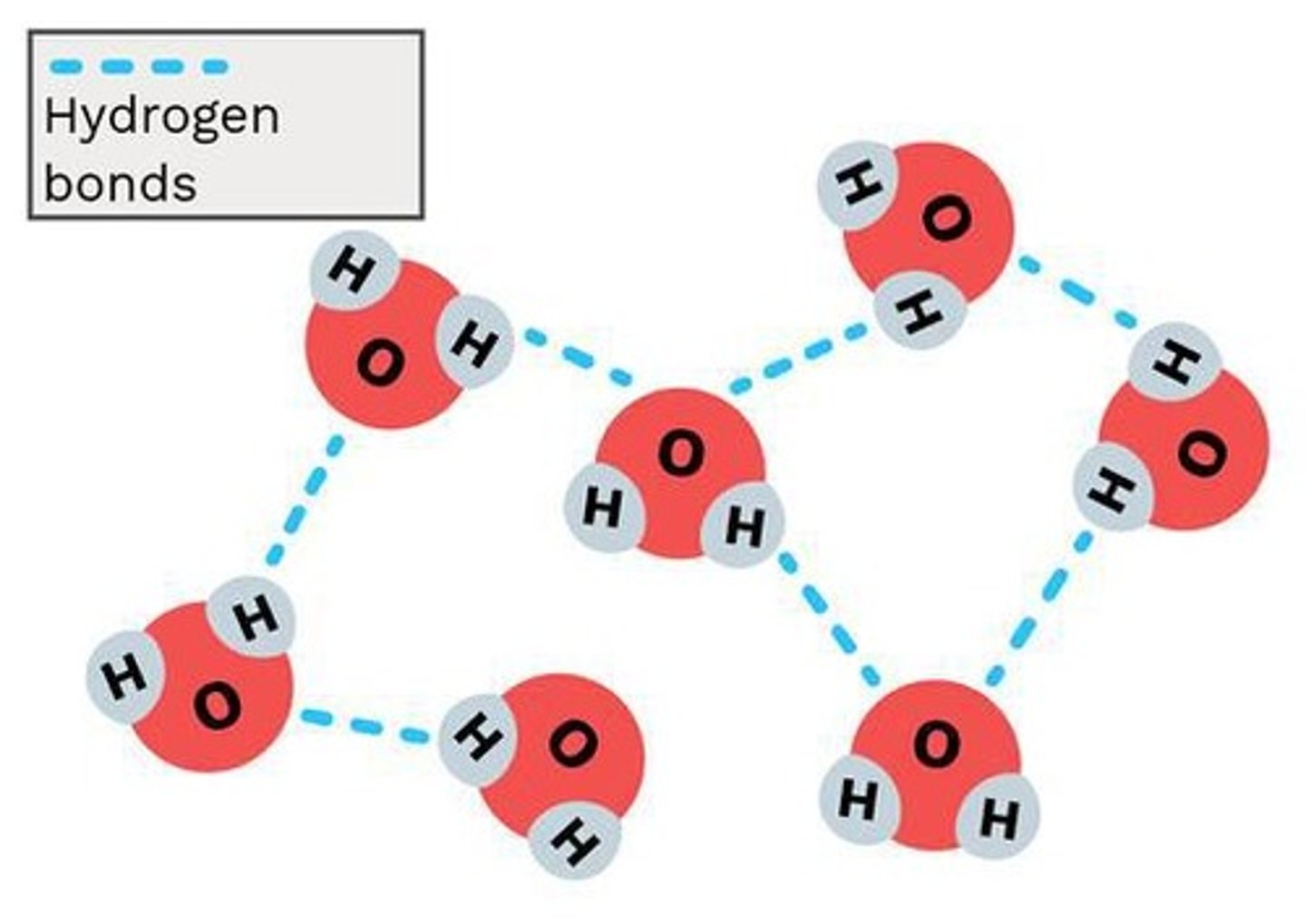
Hydrogen Bonding
H-bond donor: Electronegative atom covalently bonded to hydrogen contributed to the H-bond interaction.
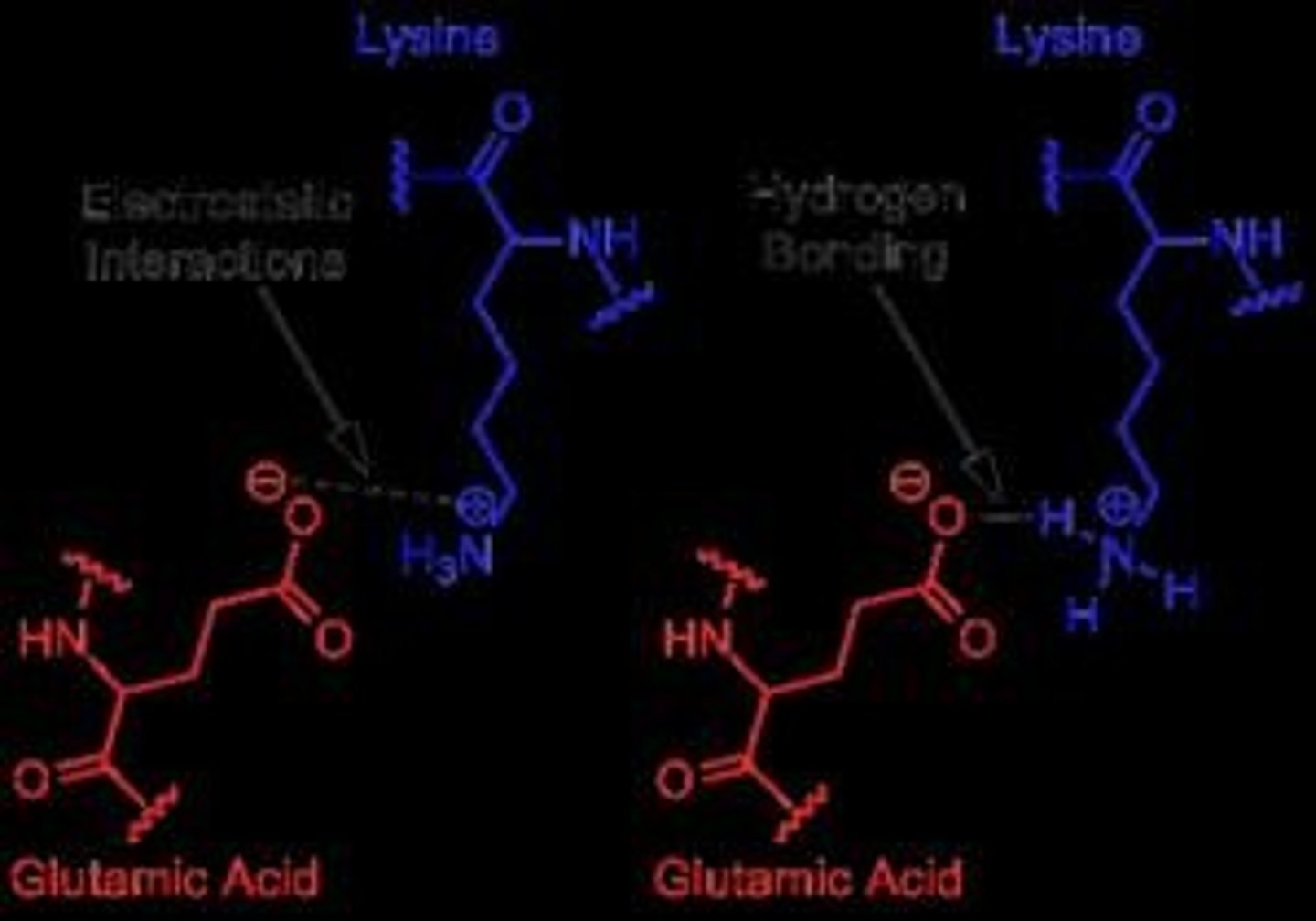
What interactions make up a salt bridge?
Combination of H-bonding and ionic interaction; a strong biochemical interaction found in proteins.
Van der Waals Forces
Weak interactions that occur between molecules.
Dipole-Dipole Interactions
Interactions between polar molecules where positive and negative ends attract.
Dipole-Induced Dipole Interactions
Interactions that occur when a polar molecule induces a dipole in a nonpolar molecule.
London Dispersion Forces
Weak attractions that occur due to temporary dipoles in molecules.
Transient dipole induction
A temporary dipole that occurs in nonpolar molecules due to fluctuations in electron distribution.
Induced Dipole-Induced Dipole Interactions
Interactions that occur between two nonpolar molecules when one induces a dipole in the other.
What are salt bridges?
A strong biochemical interaction that provides structure to proteins.
Molecular Level
Refers to interactions at the level of molecules.
Cellular Level
Refers to interactions at the level of cells.
Organismal Level
Refers to interactions at the level of entire organisms.
Chemical interactions vary in strength
Different types of chemical interactions have varying degrees of strength.