general chemistry 1: atomic strucutre
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms

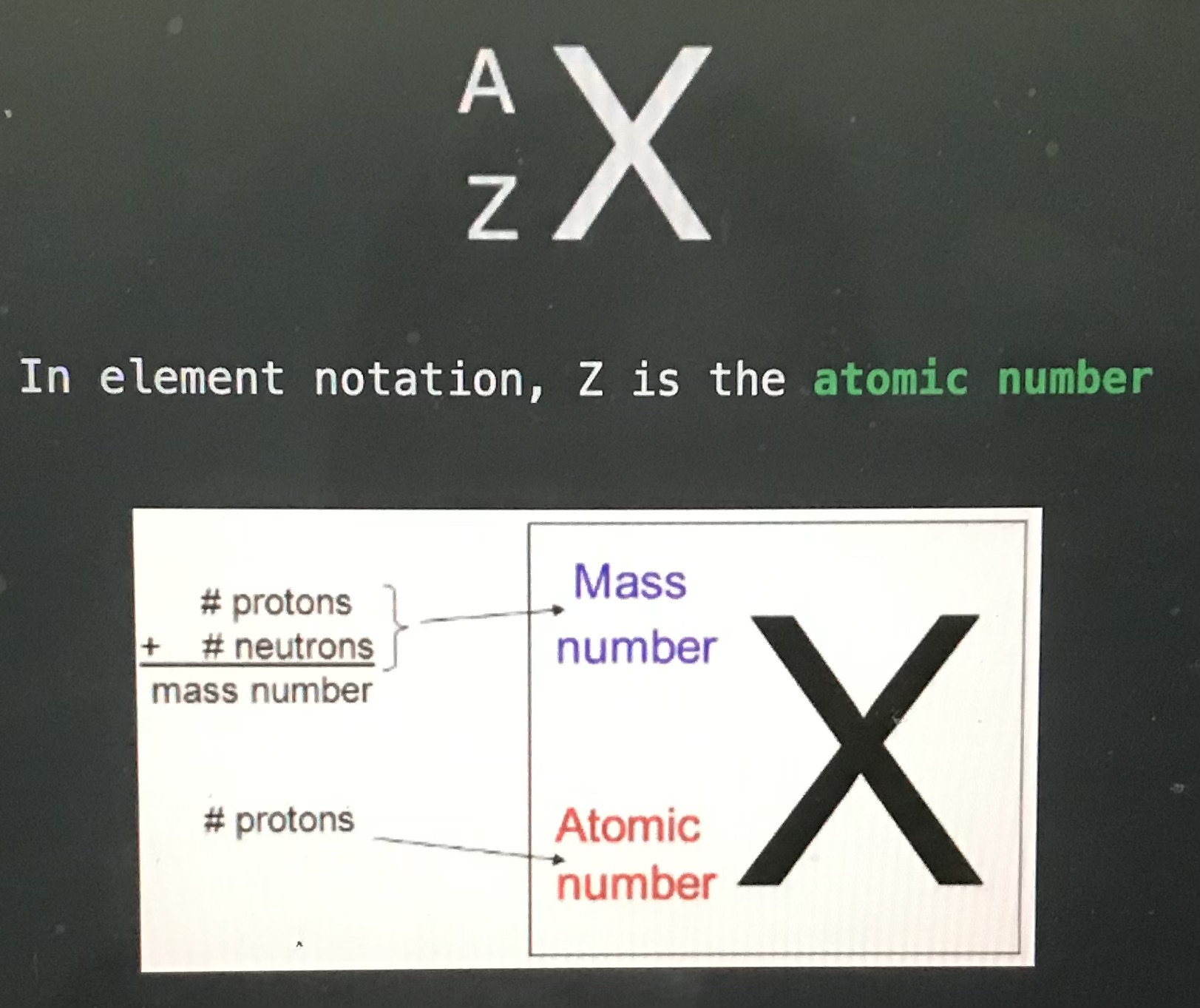
In element notation A is the
Mass number
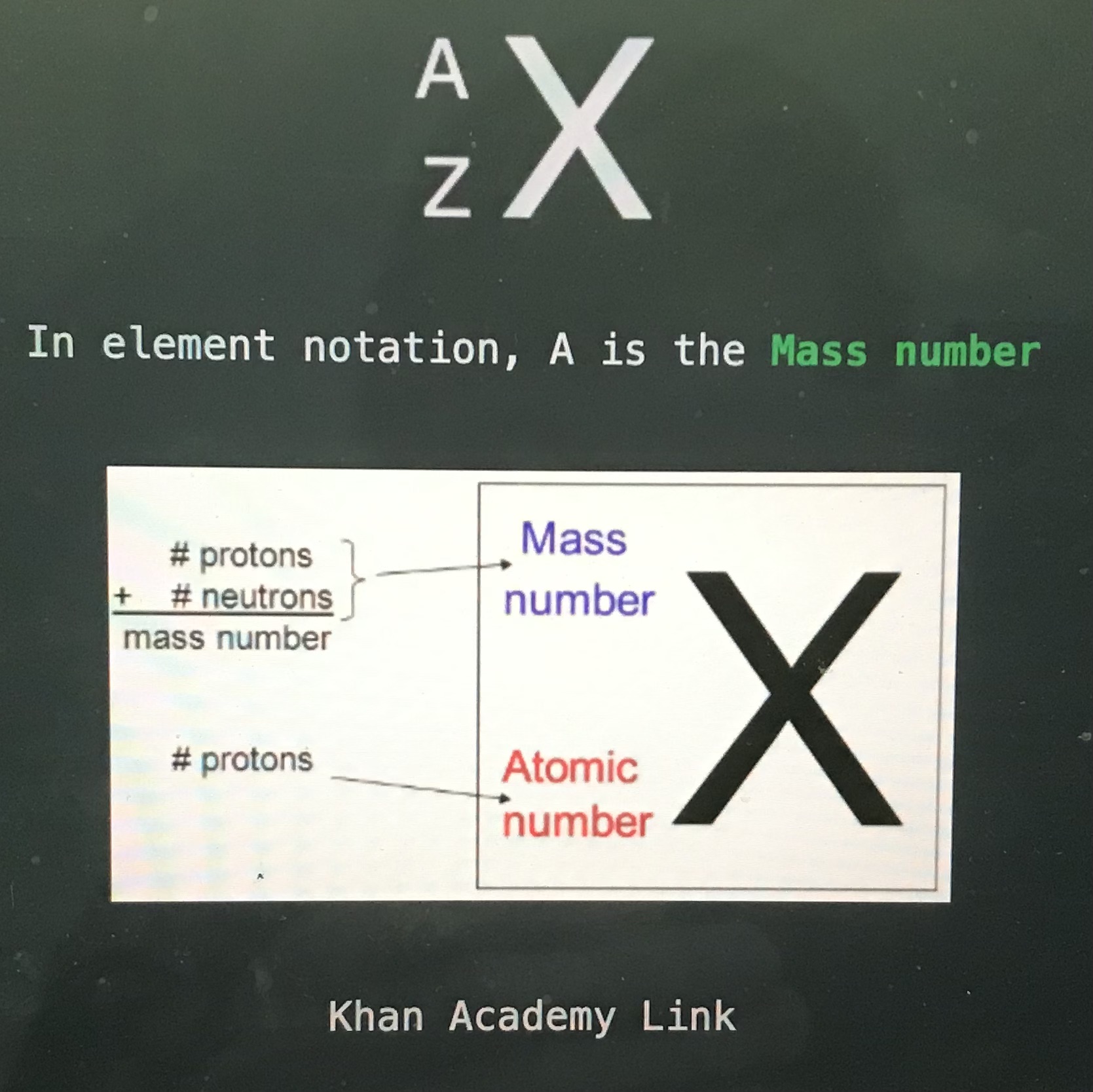

In element notation, Z is the
Atomic number

Blank is the weighted average of the masses of an elements isotopes
Atomic weight

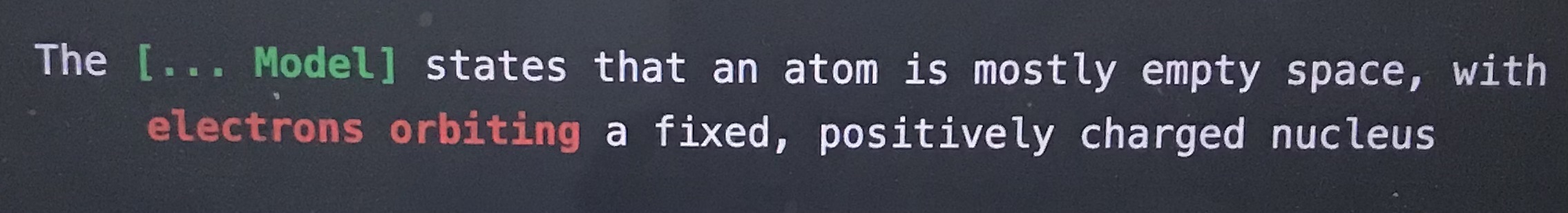
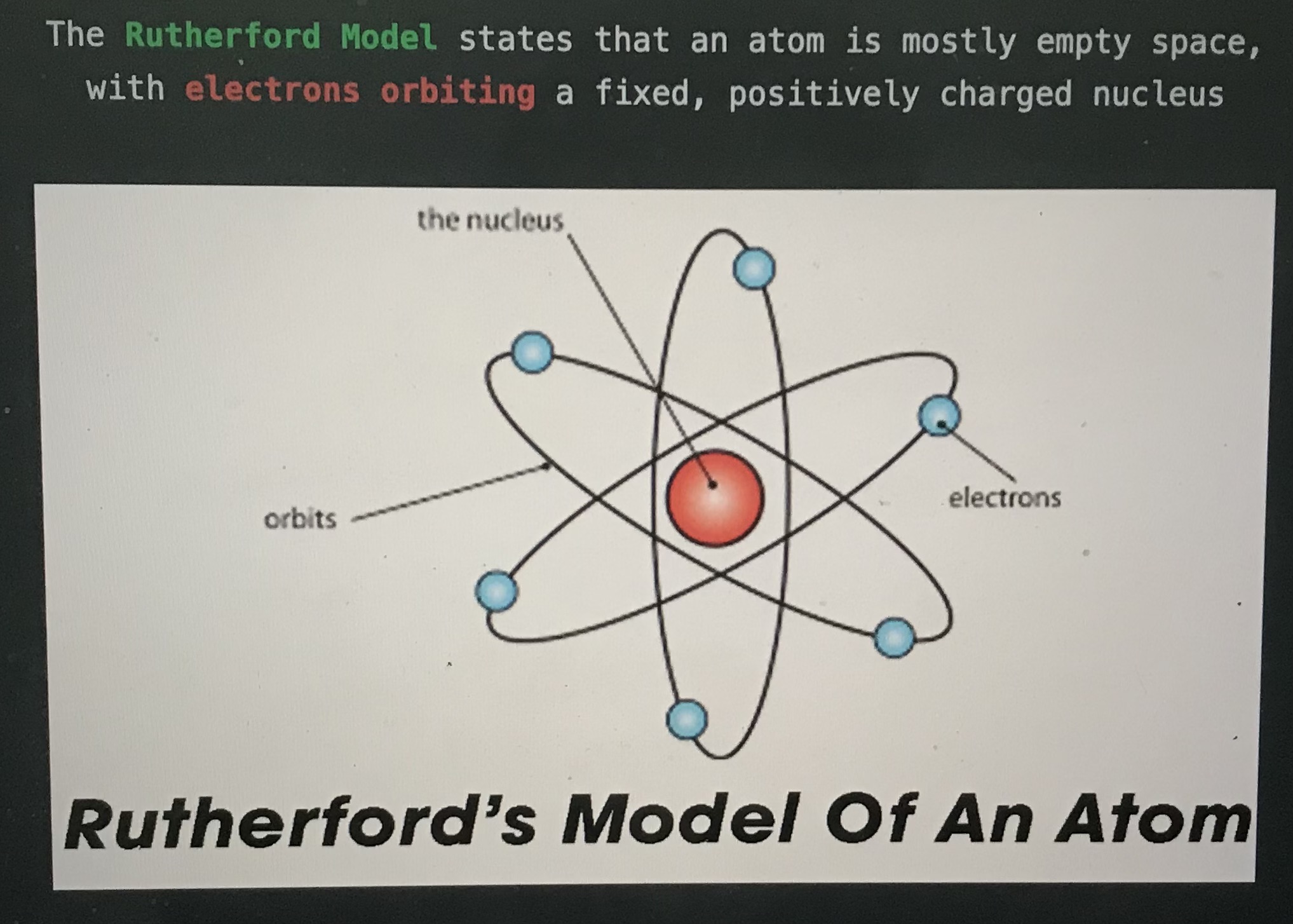
The blank model states that an atom is mostly empty space with electrons orbiting a fixed, positively charged nucleus
Rutherford model
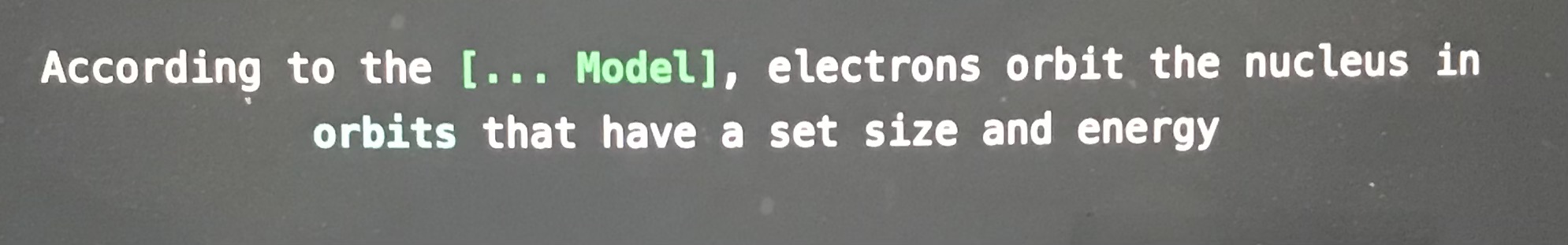
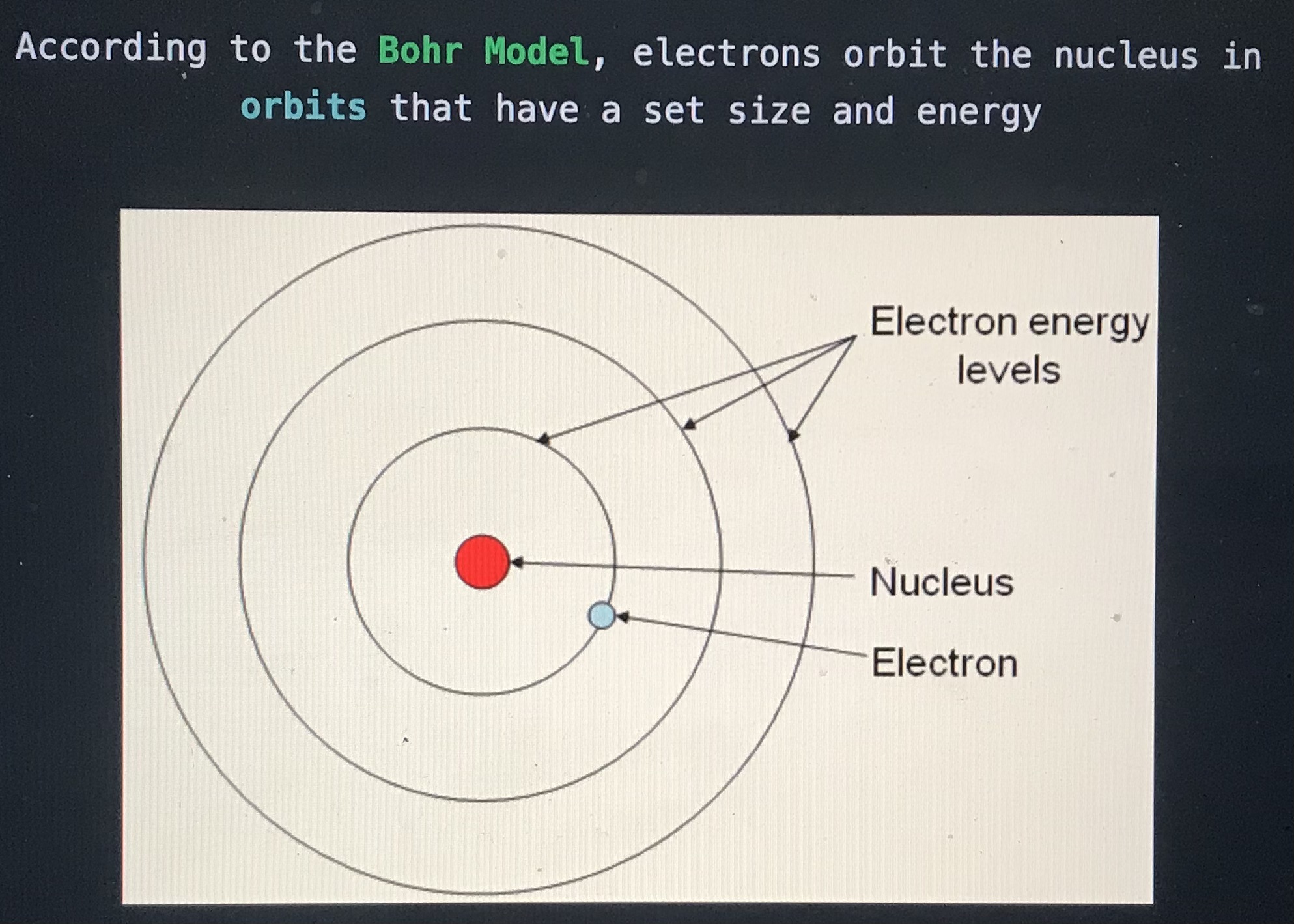
According to the blank model, electrons orbit the nucleus in orbits that have a set size and energy
Bohr model

The blank principle states that it is impossible to know the momentum and position of an electron simultaneously
Heisenberg uncertainty Principle


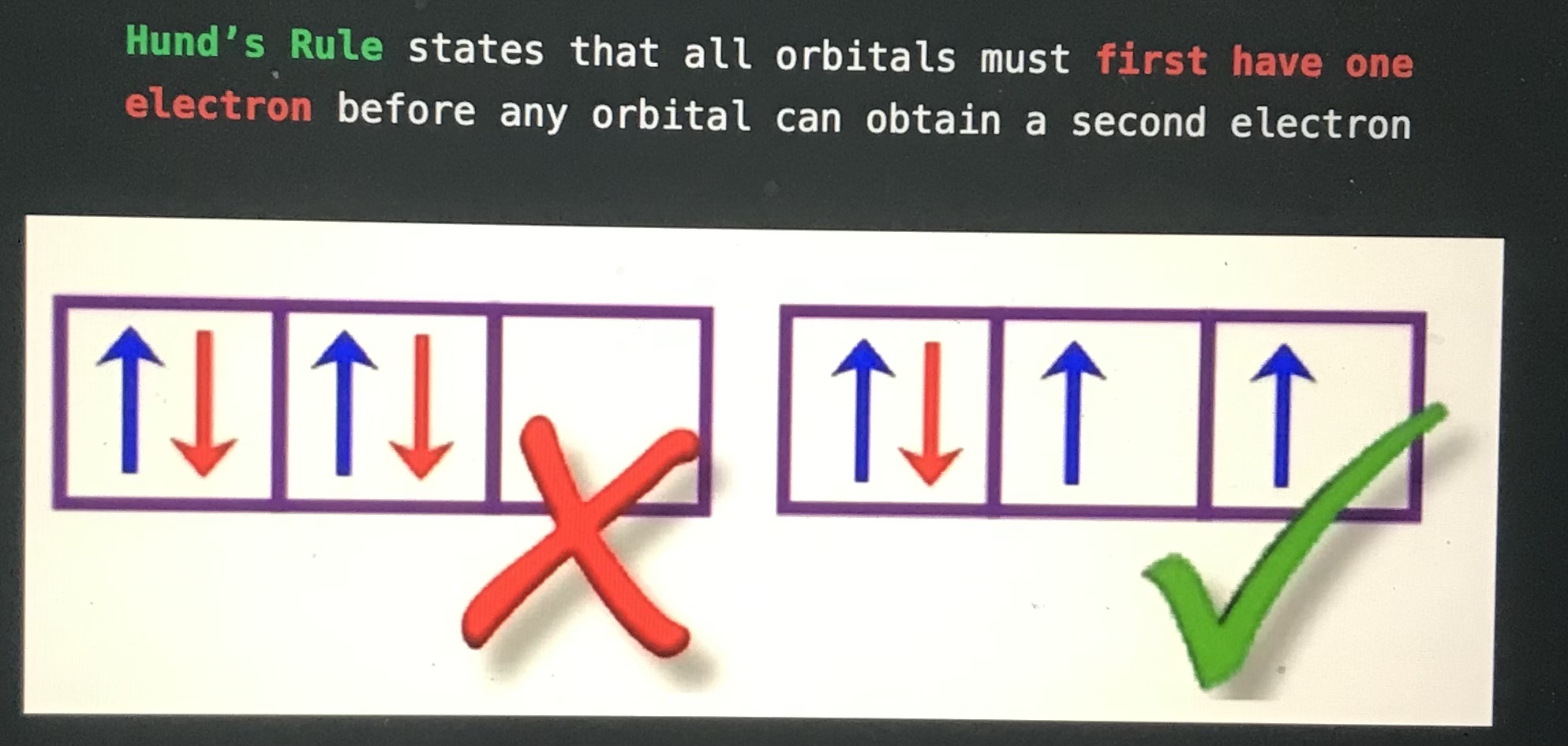
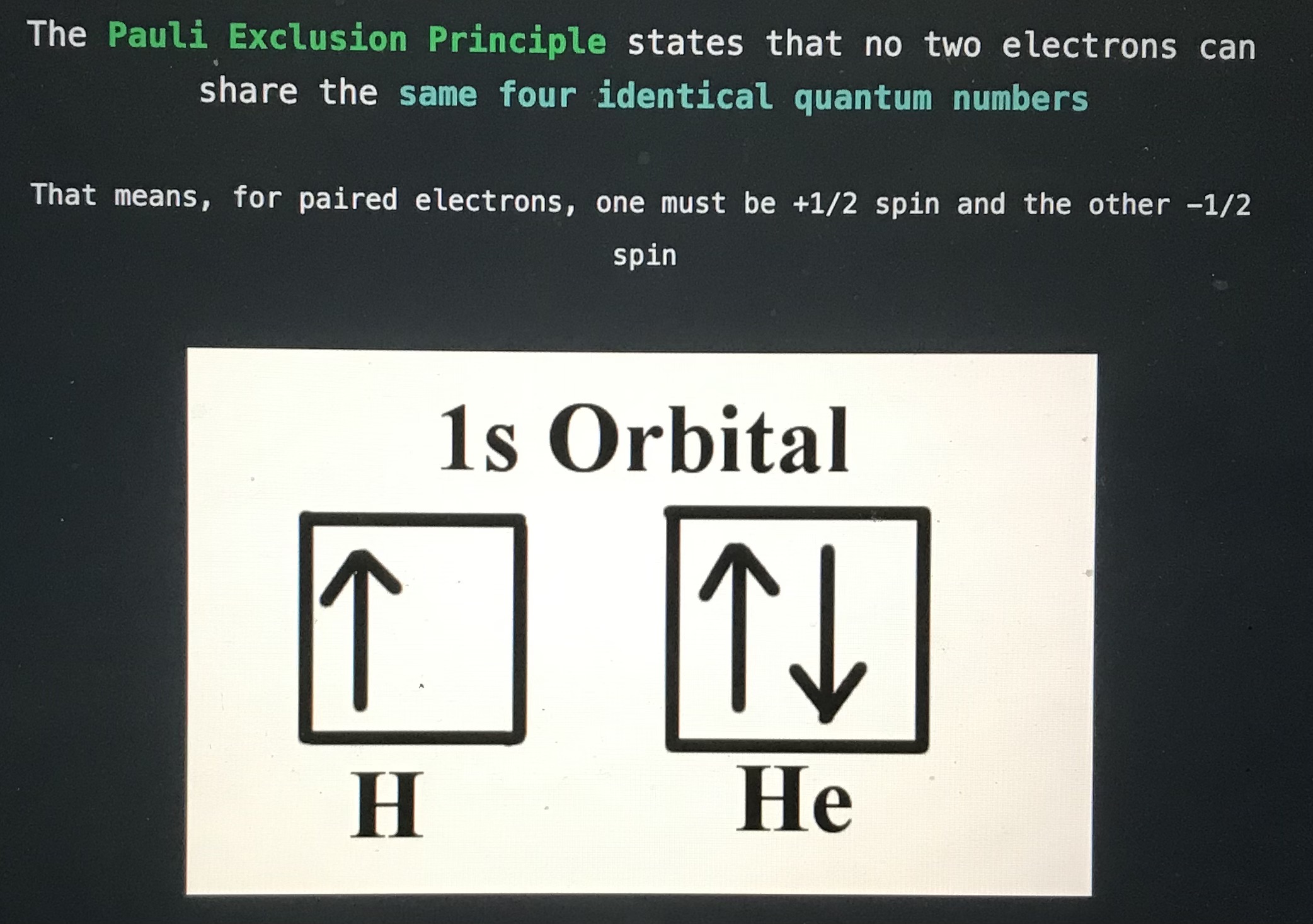
Blank states that all orbitals must first have one electron before any orbitals can obtain a second electron
Hund's Rule

The blank principle states that no two electrons can share the same for identical quantum numbers
Pauli Exclusion Principle
Avogadro’s number =
6.022 x 10²³ particles per 1 mole

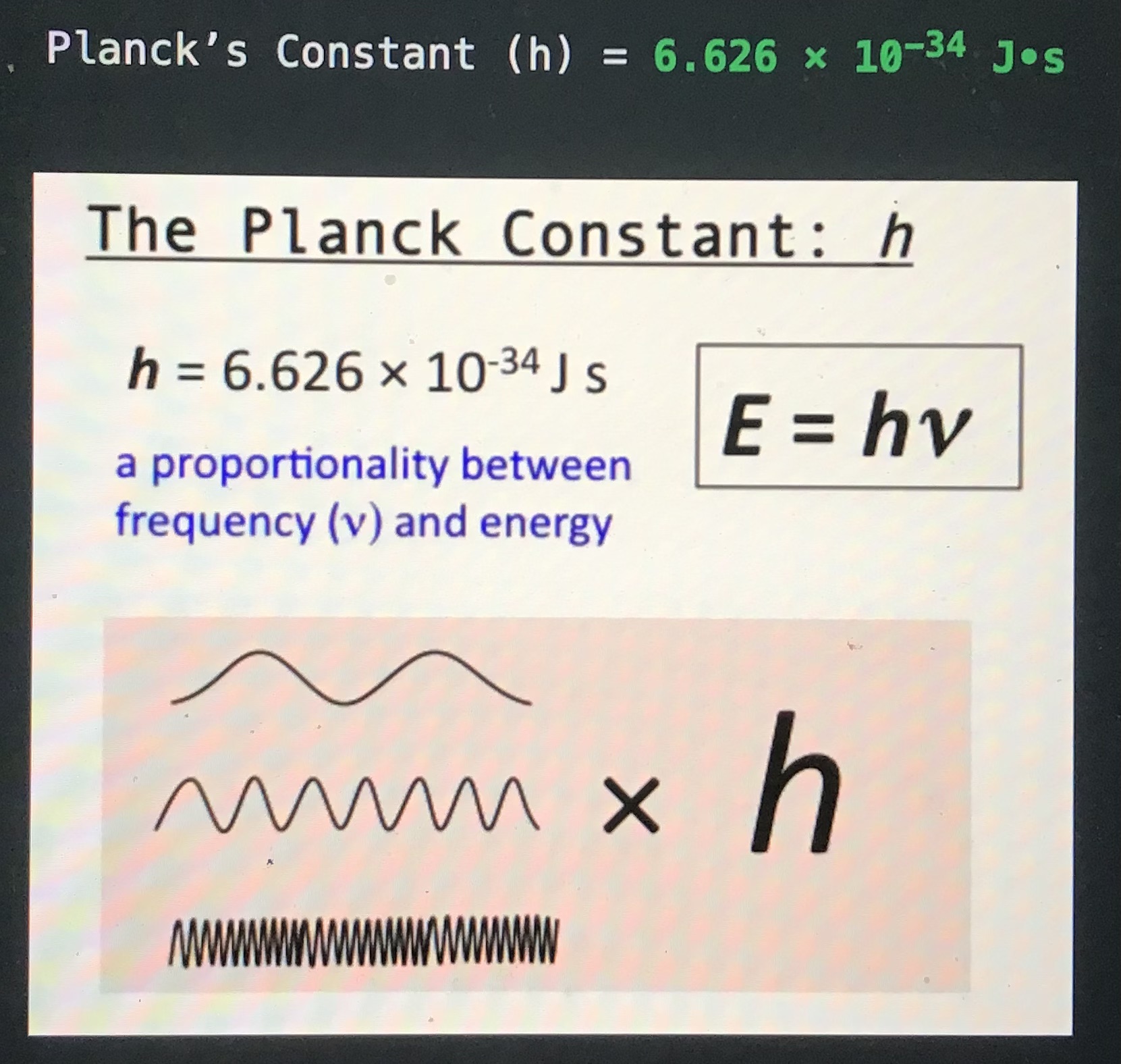
Planck’s constant (h) =
6.626 x 10⁻³⁴ Js
Speed of light ( c ) =
3.00 x 10⁸ m/s
Give the equation for energy of a photon:
f = photon frequency
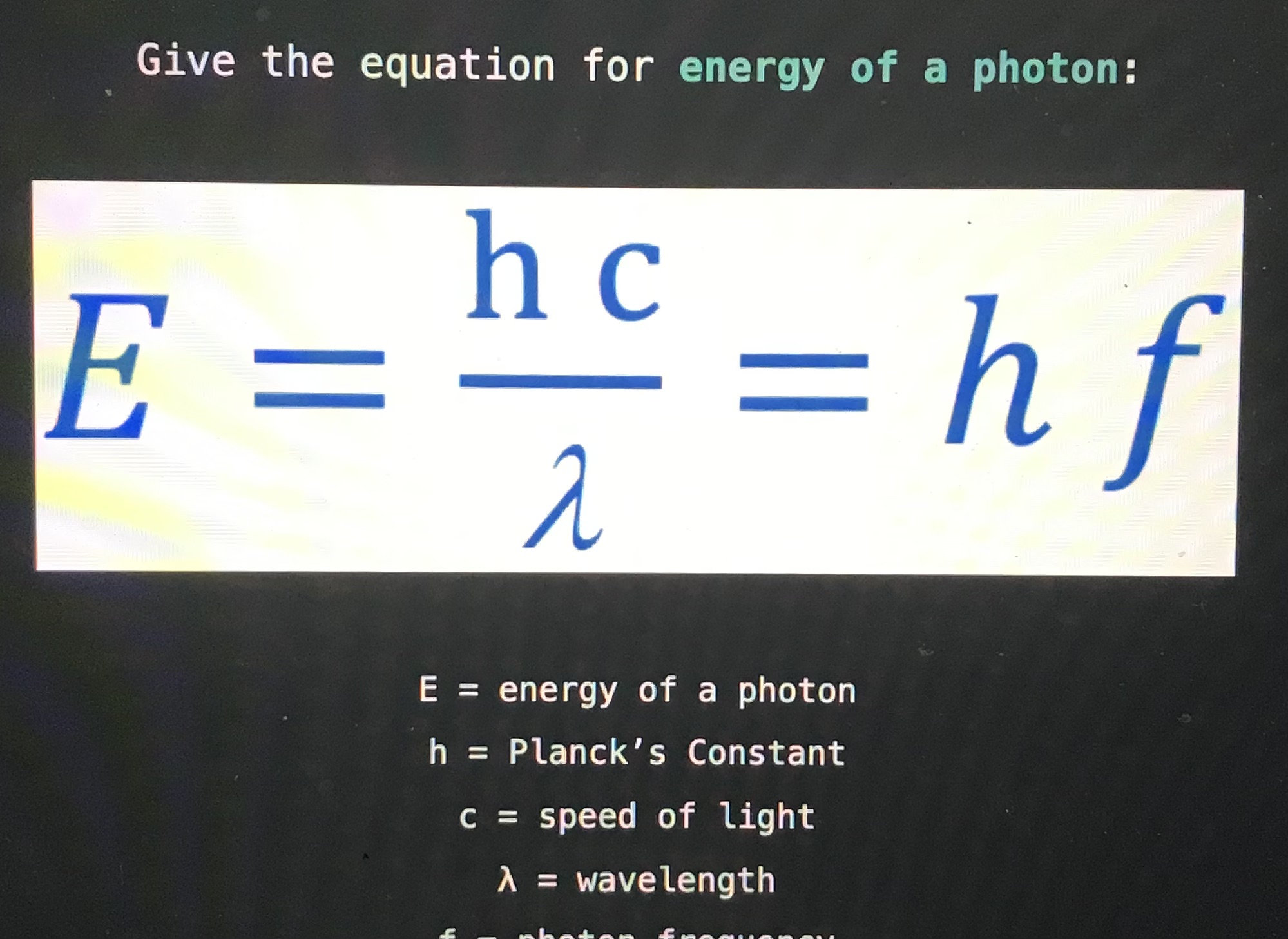
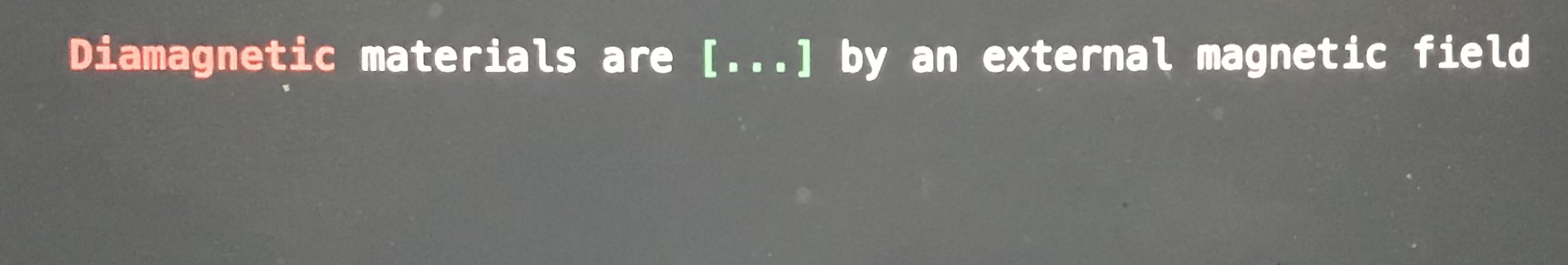
Diamagnetic materials are blank by an external magnetic field
Repelled
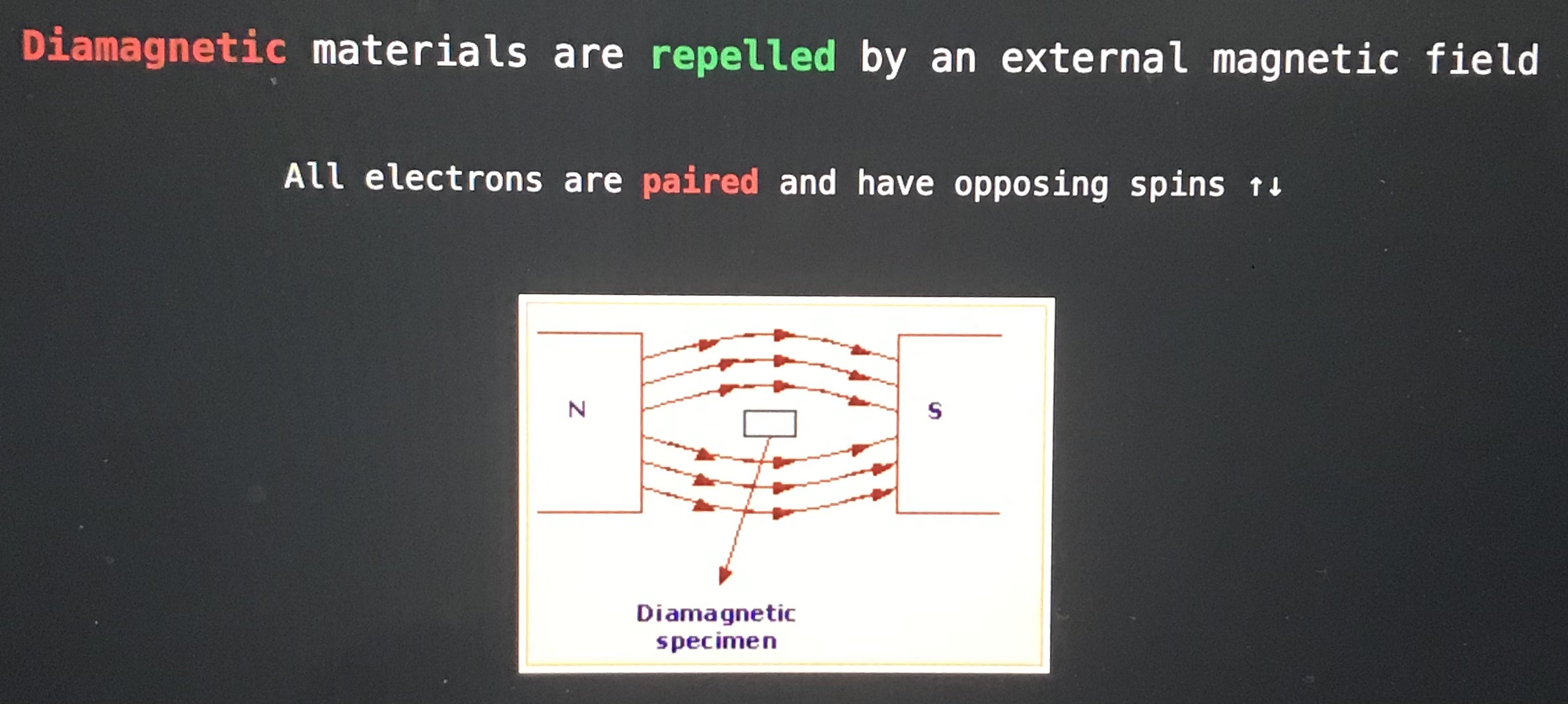

Paramagnetic material are blank an external magnetic field
attracted by (pulled into)

He is (diamagnetic or paramagnetic)
diamagnetic

Li is (diamagnetic or paramagnetic)
paramagnetic

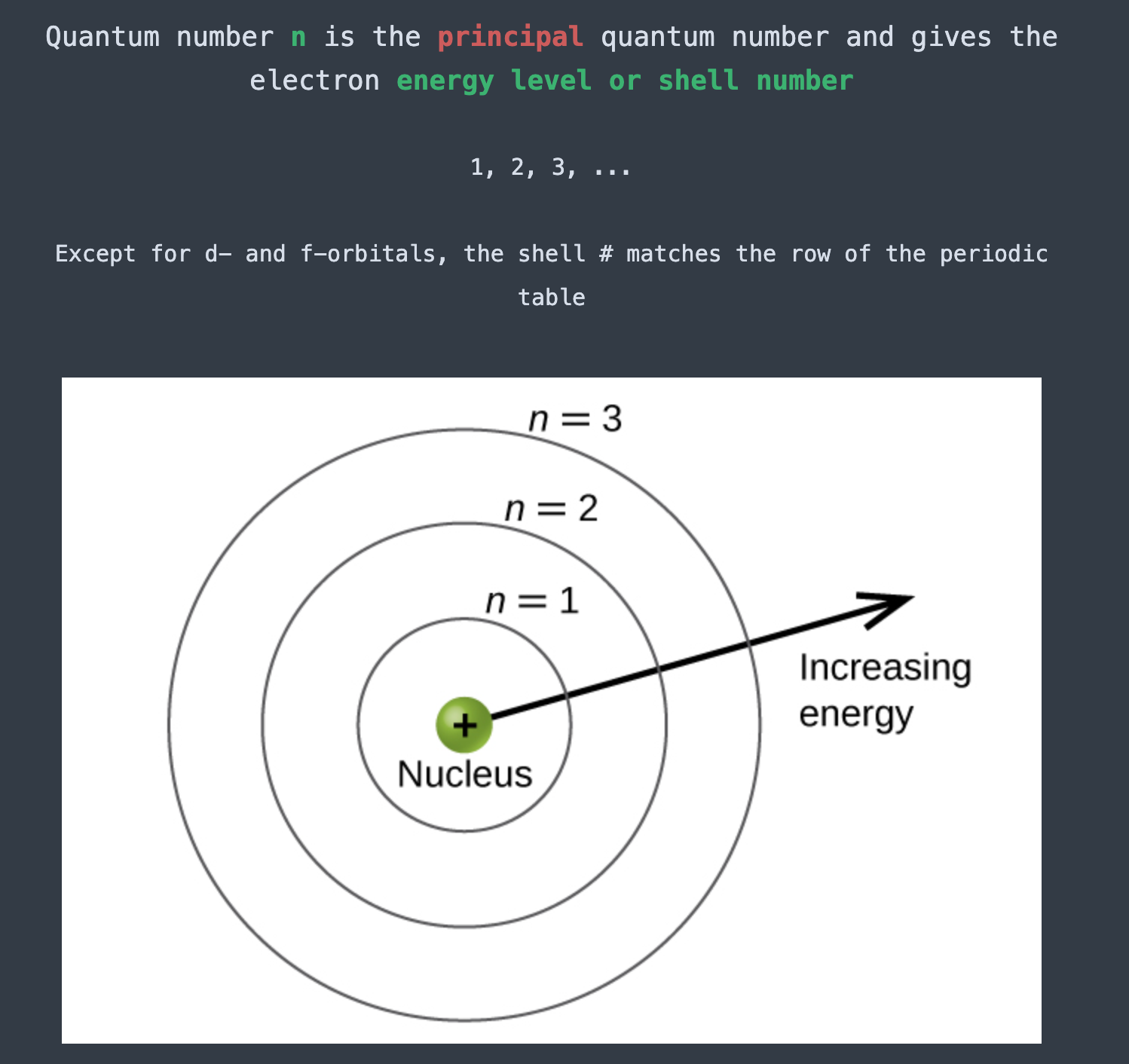
Quantum number blank is the principal quantum number, and gives the electron blank
n; energy level or shell number
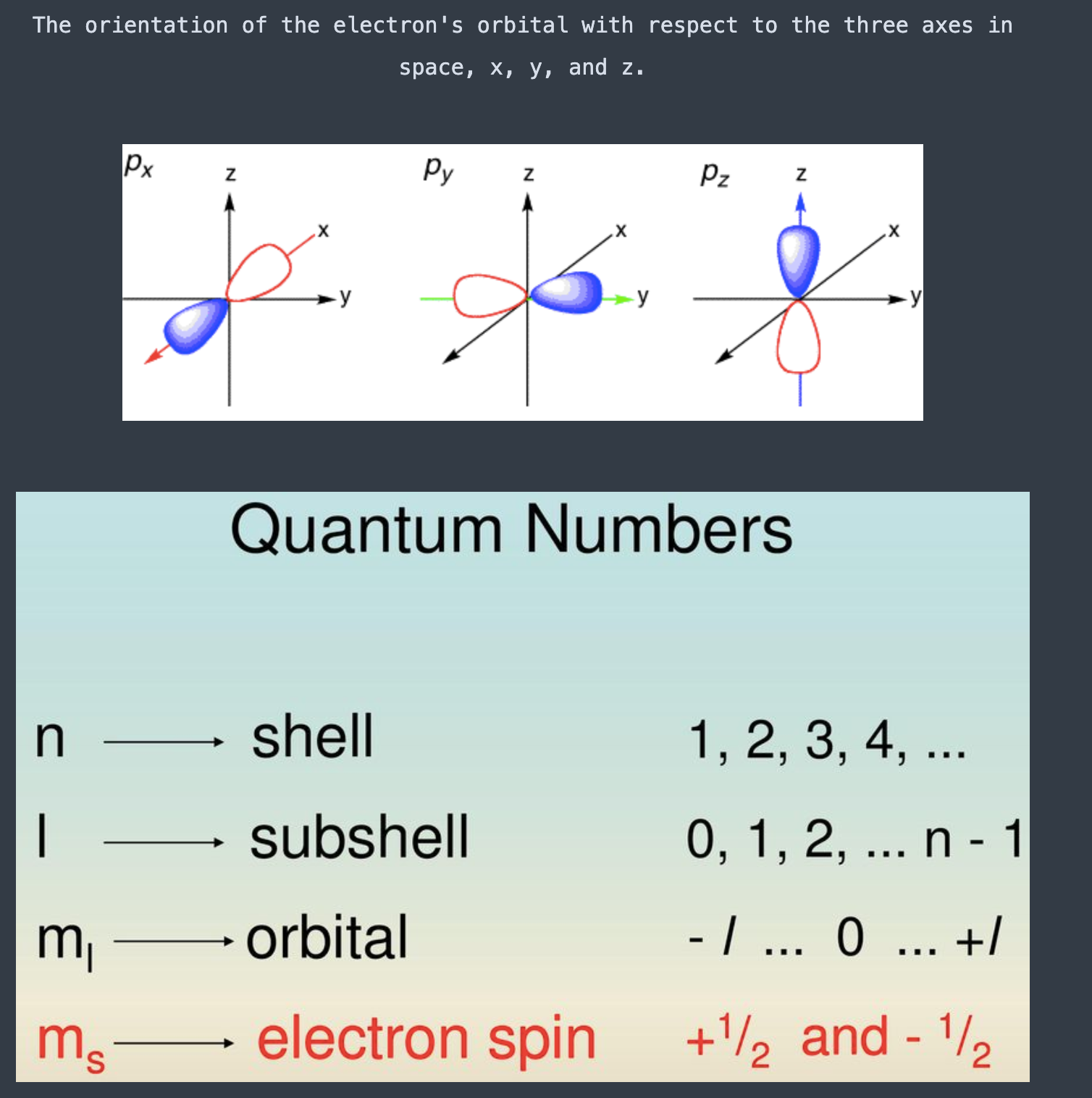
Quantum number [...] is the azimuthal quantum number and gives the [...] of an orbital
l; shape

Quantum number [...] is the magnetic quantum number and gives the orbital [...]
ml ; sub-type
Quantum number ml is the magnetic quantum number and gives the orbital sub-type
Integers -l to +l
The orientation of the electron's orbital with respect to the three axes in space, x, y, and z.
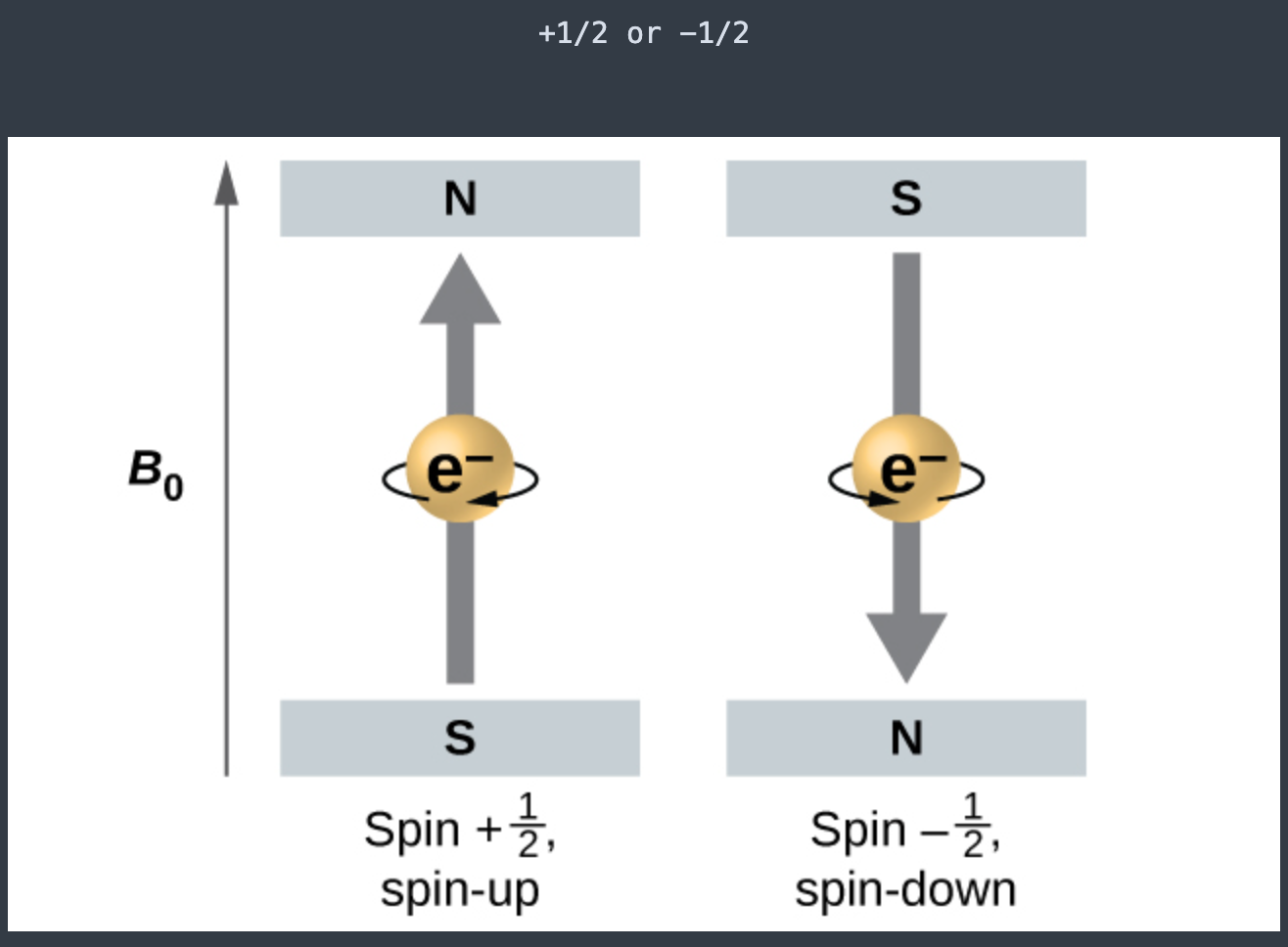
Quantum number [...] is the spin quantum number and gives the [...] of the electron
Quantum number ms is the spin quantum number and gives the electronic spin of the electron
+ ½ -1/2
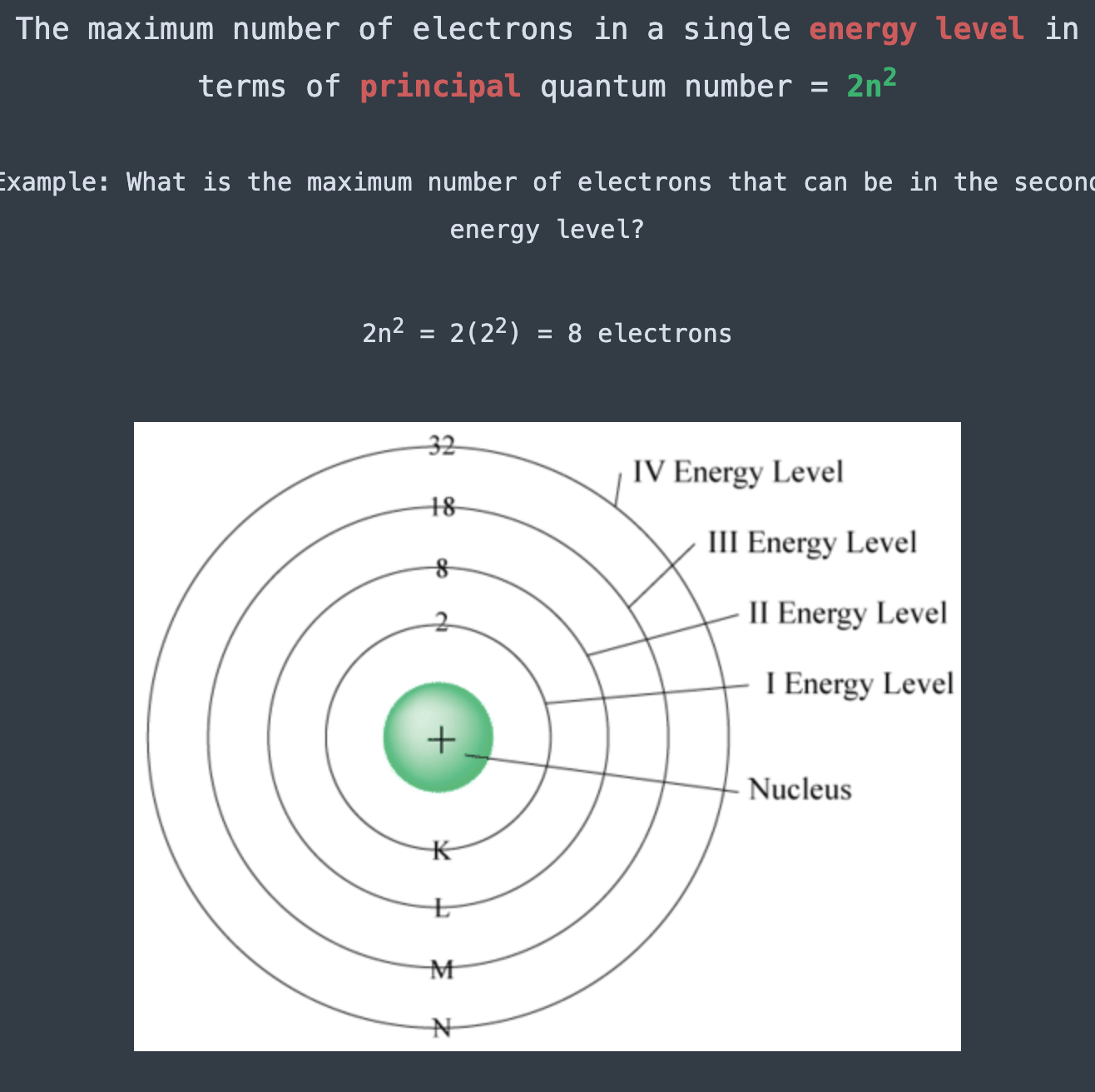
The maximum number of electrons in a single energy level in terms of principle quantum number =
2n², where n is the principal quantum number.
The maximum number of electrons in a single subshell in terms of the azimuthal quantum number = [...]
4l+2 where l is the azimuthal quantum number.

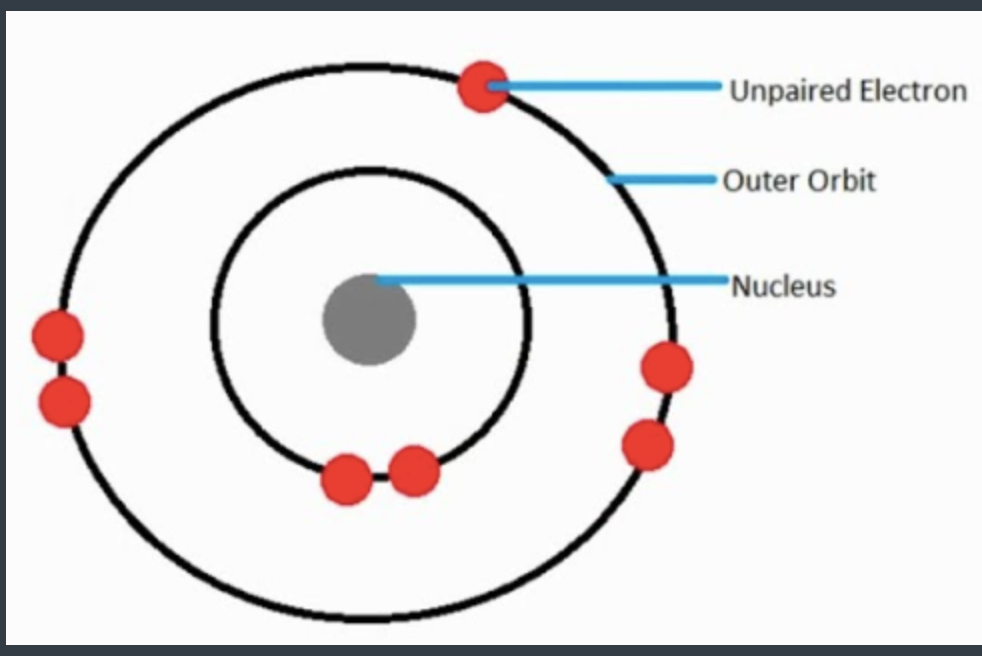
A/an [...] is an atom or molecule with an unpaired electron
free radical
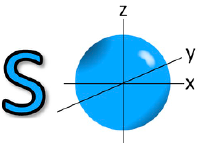
![<p><span>he Azimuthal quantum number for this orbital is </span><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/2ca34d71-c17a-457b-a5b2-ed904b1149b6.png)
he Azimuthal quantum number for this orbital is [...]
0 = s orbital
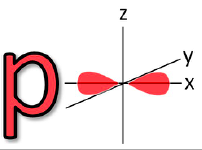
![<p><span>The Azimuthal quantum number for this orbital is </span><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/0c3e6a06-3baa-4736-a8f5-23ee67d01c5c.png)
The Azimuthal quantum number for this orbital is [...]
1 = p orbital
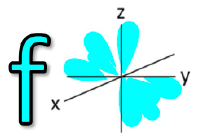
![<p><span>The Azimuthal quantum number for this orbital is </span><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/4a864439-9caa-4cac-a655-578e864742b1.png)
The Azimuthal quantum number for this orbital is [...]
2 = d orbital
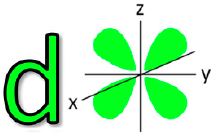
![<p>T<span>he Azimuthal quantum number for this orbital is </span><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/a679a5b9-b5af-4196-bf64-e0e4080c7f23.png)
The Azimuthal quantum number for this orbital is [...]
3 = f orbital
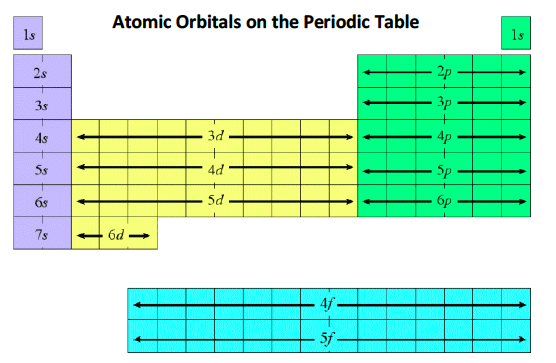
![<p>Give the <strong><u>principal</u></strong> and <strong>azimuthal</strong> quantum number labels for the periodic table</p><p><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/5df5cf63-108f-4637-9554-b69339df3c1d.png)
Give the principal and azimuthal quantum number labels for the periodic table
[...]
Principal (n) quantum number = 1, 2, 3, ...
Azimuthal (l) quantum number = 0, 1, 2, …, n-1
The [... Principle] states that electrons will fill the lower energy levels before moving to higher energy orbitals
aufbau principle