equilibria
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
what is le Chatelier’s principle?
when an external change is made to a system in equilibrium, the system responds to minimise the effect
what is the equation linking Kp and Kc
Kp = Kc (RT)∆n
show how PV = nRT goes to Kp = Kc (RT)∆n
how does ∆G vary with extent of reaction?
show graph

why is activity dimensionless?
it is measured relative to a standard
how to find activity of a gas?
partial pressure / p⦵ (1 bar or 105 Pa)
how to find activity of solution?
[A] / [A]⦵ = [A] / 1 mol dm-3
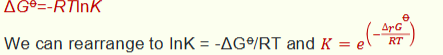
how does this equation show that K is constant with pressure but not with temperature?
∆G⦵ is defined as being at 1 bar so is constant
∆G⦵ changes with temperature so K does as well
how to convert to van ‘t Hoff from ∆G?
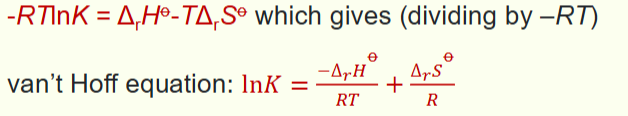
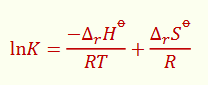
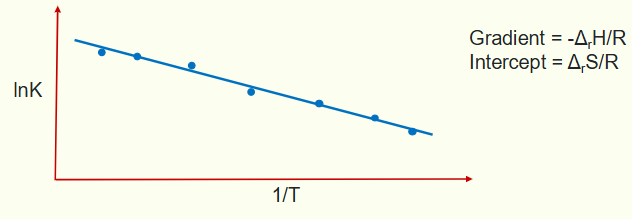
for the van ‘t Hoff equation, what is the graph? what is the gradient and intercept?