Separation of ternary mixtures
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
what is separation
isolating or purifying components of a mixture based on differences in physical or chemical properties
sieving?
separating particles of different sizes by allowing the smaller particles to fall through holes in a container
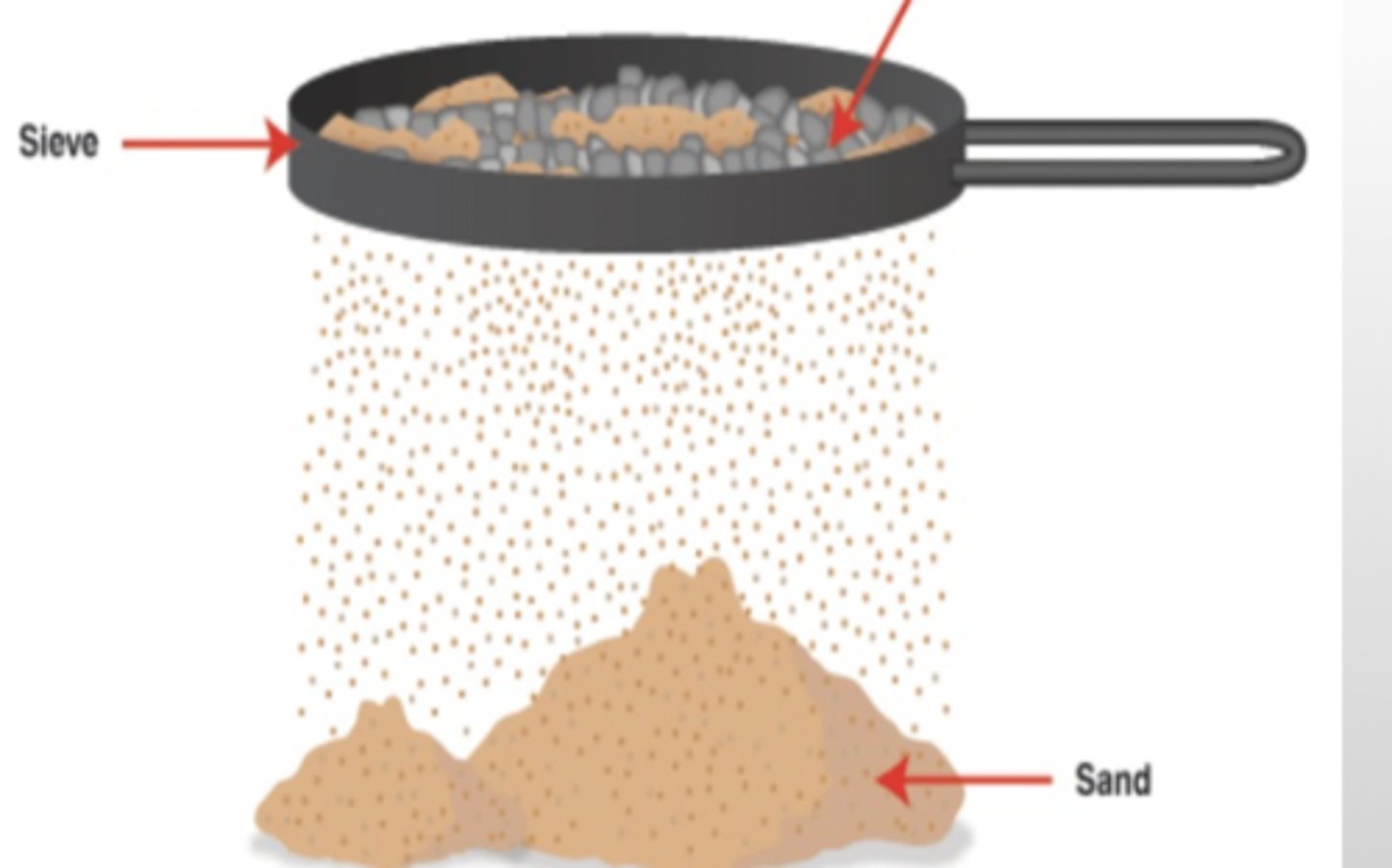
techniques of separation
decanting, filtration, crystallization, liquid extraction, distillation, chromatography, sublimation, centrifugation

decanting
Pouring liquid off the top when sediment has settled to the bottom of the container
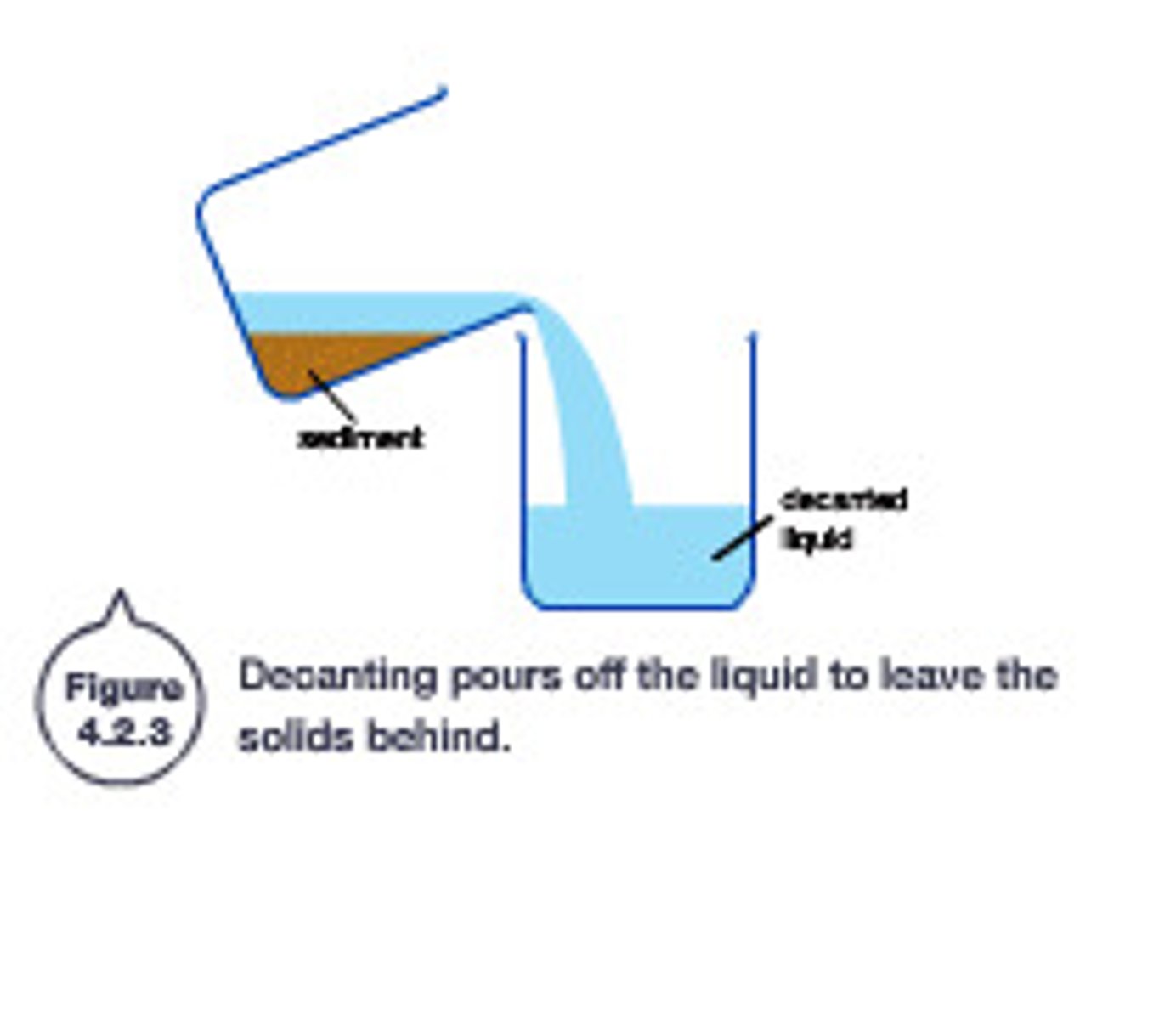
decanting is based on...
differences in the states of matter
filtration
the process that separates a solid from the liquid in a heterogeneous mixture
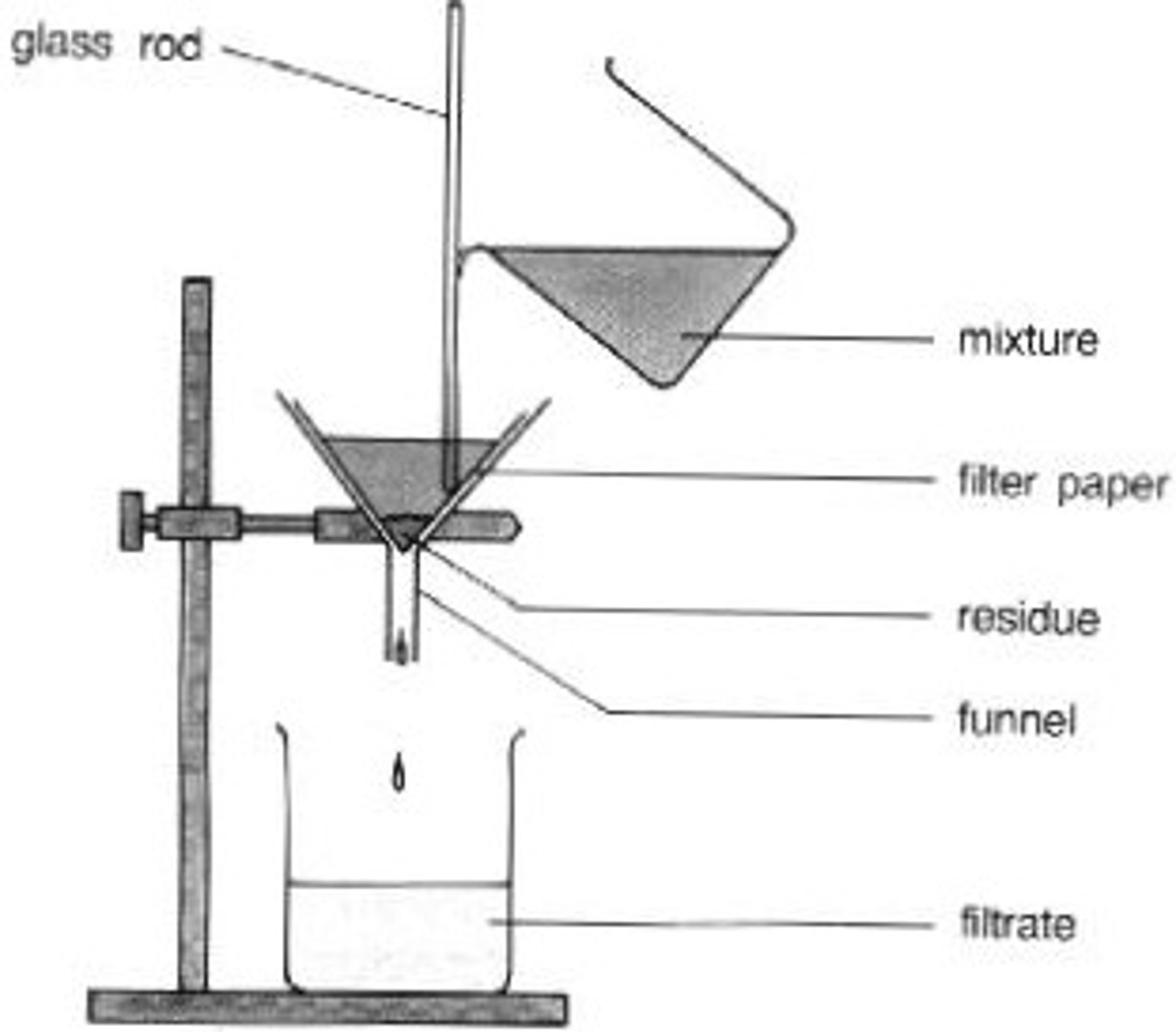
suction filtration
process of isolating pure solid from liquid (filtrate) using vacuum

water aspirator
For creating suction through water pressure

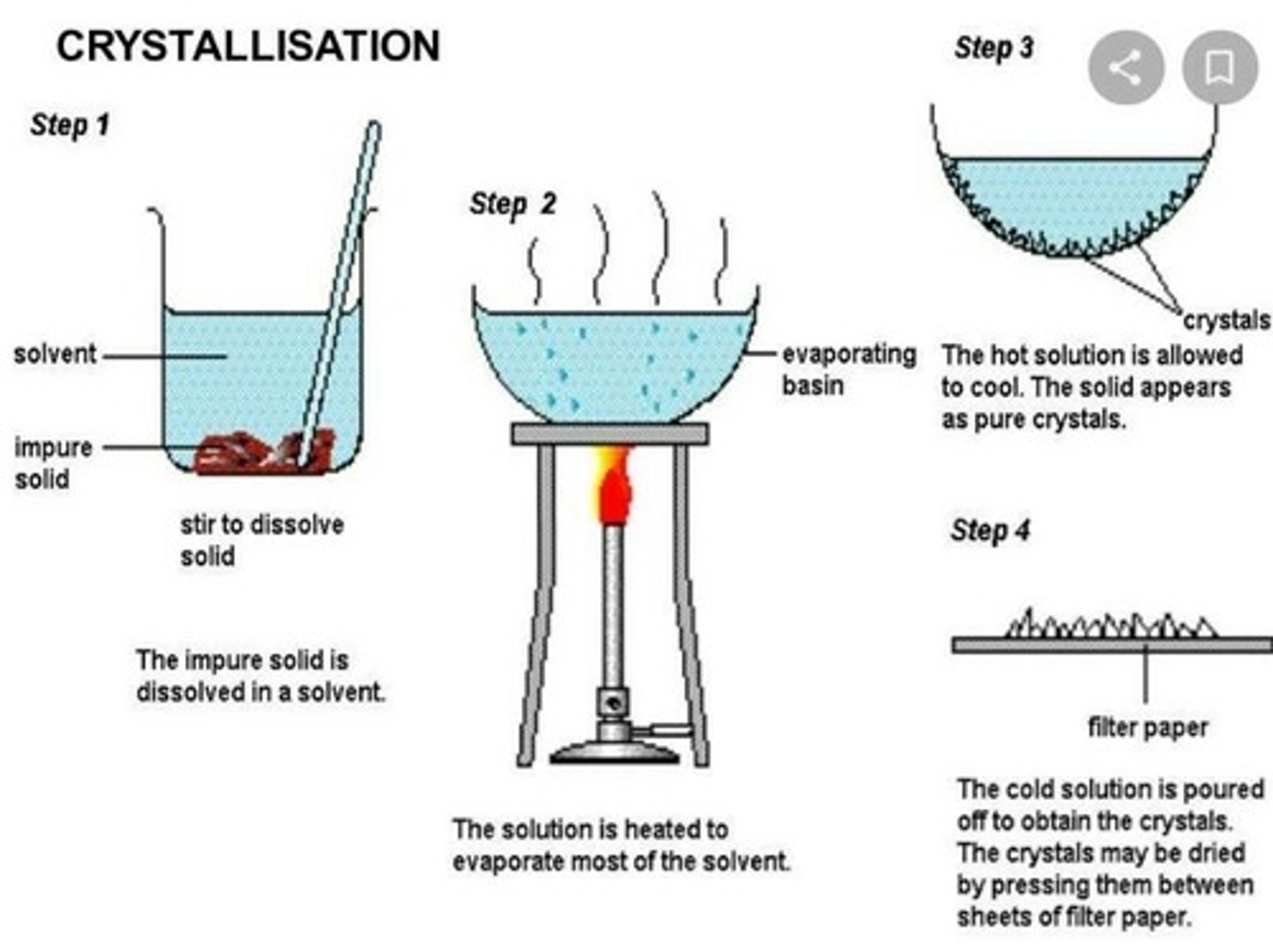
crysallization based on
differences in the solubilities of the particles in the solution
crystallization
the formation of pure solid particles of a substance from a solution containing the dissolved substance by allowing it to cool; removes impurities
liquid extraction
Two immiscible liquid phases, solute transferred between phases
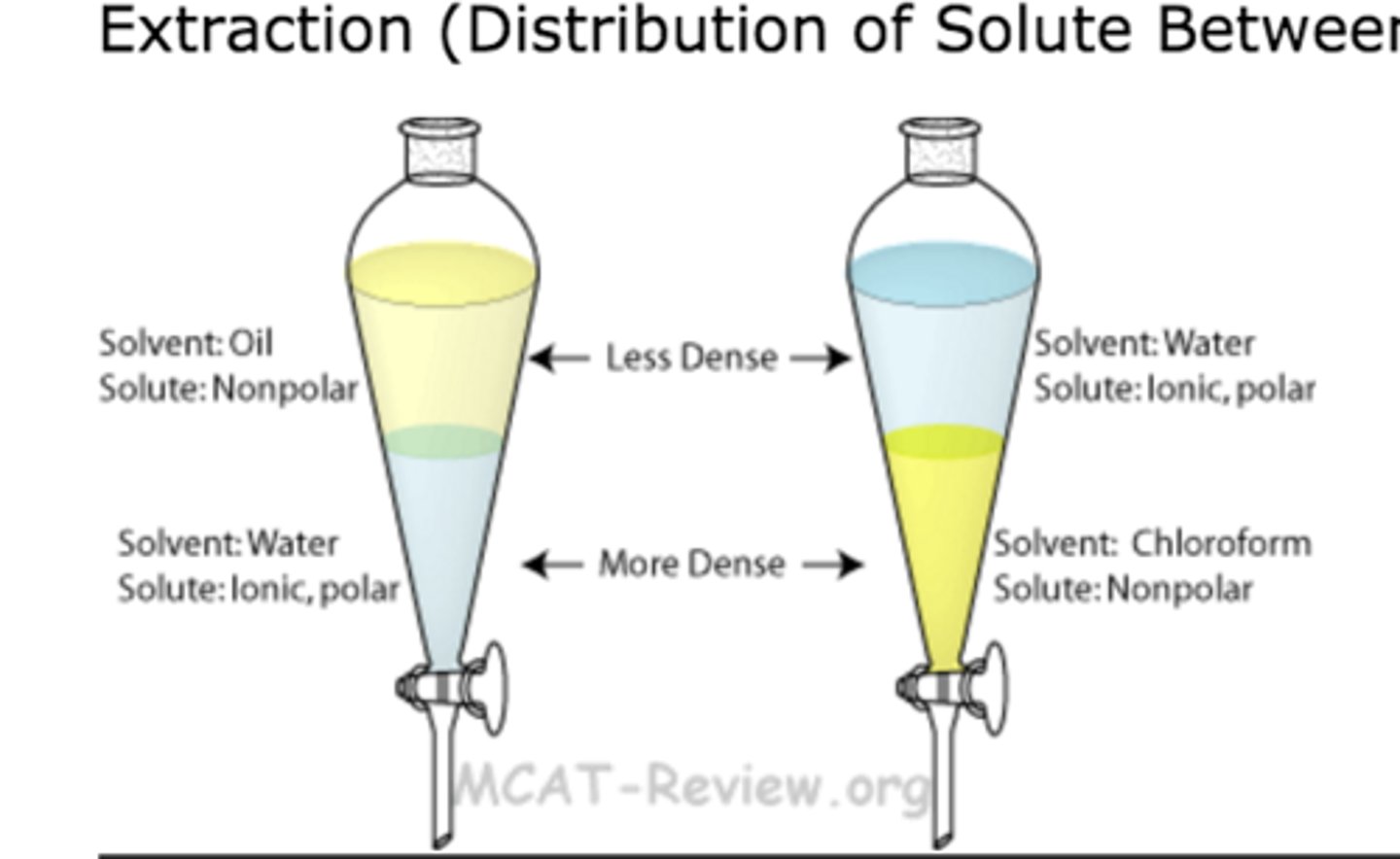
liquid extraction is based on
differences in solubilities and polarities of a compound to be separated

top layer in extractions
has a lower density
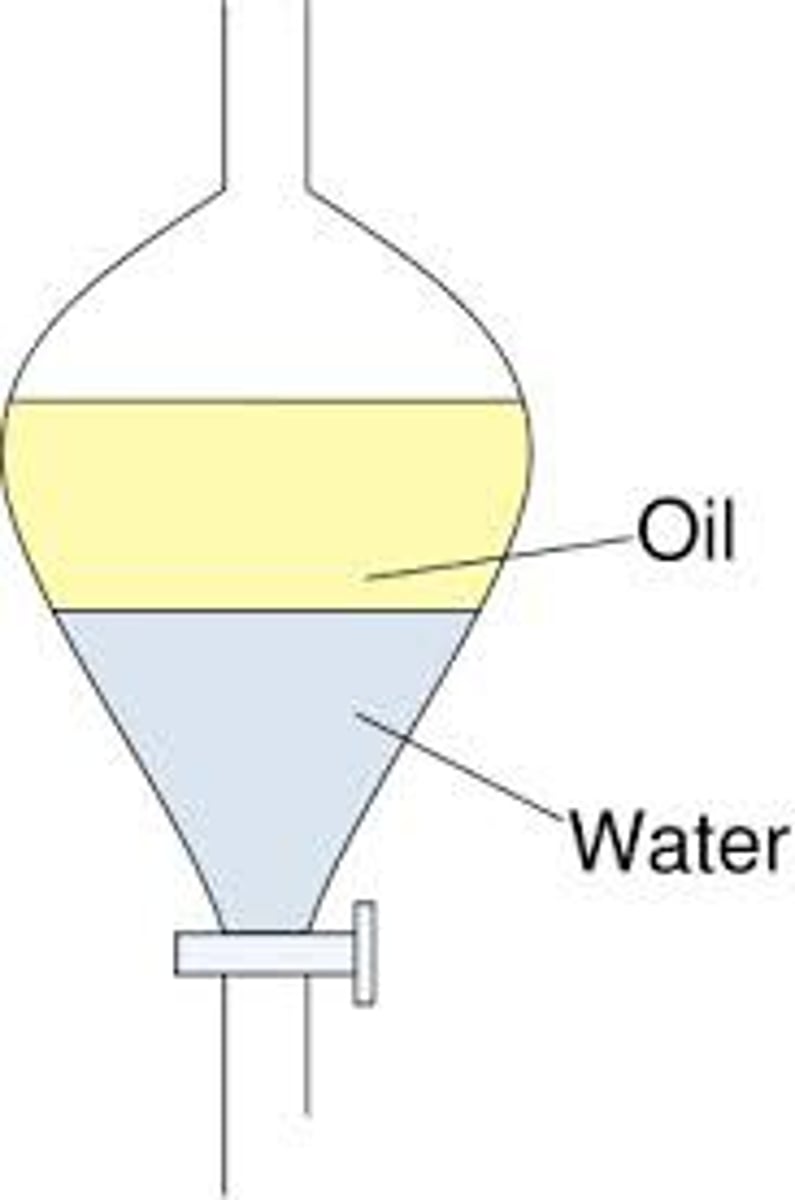
bottom layer in extractions
has a larger density
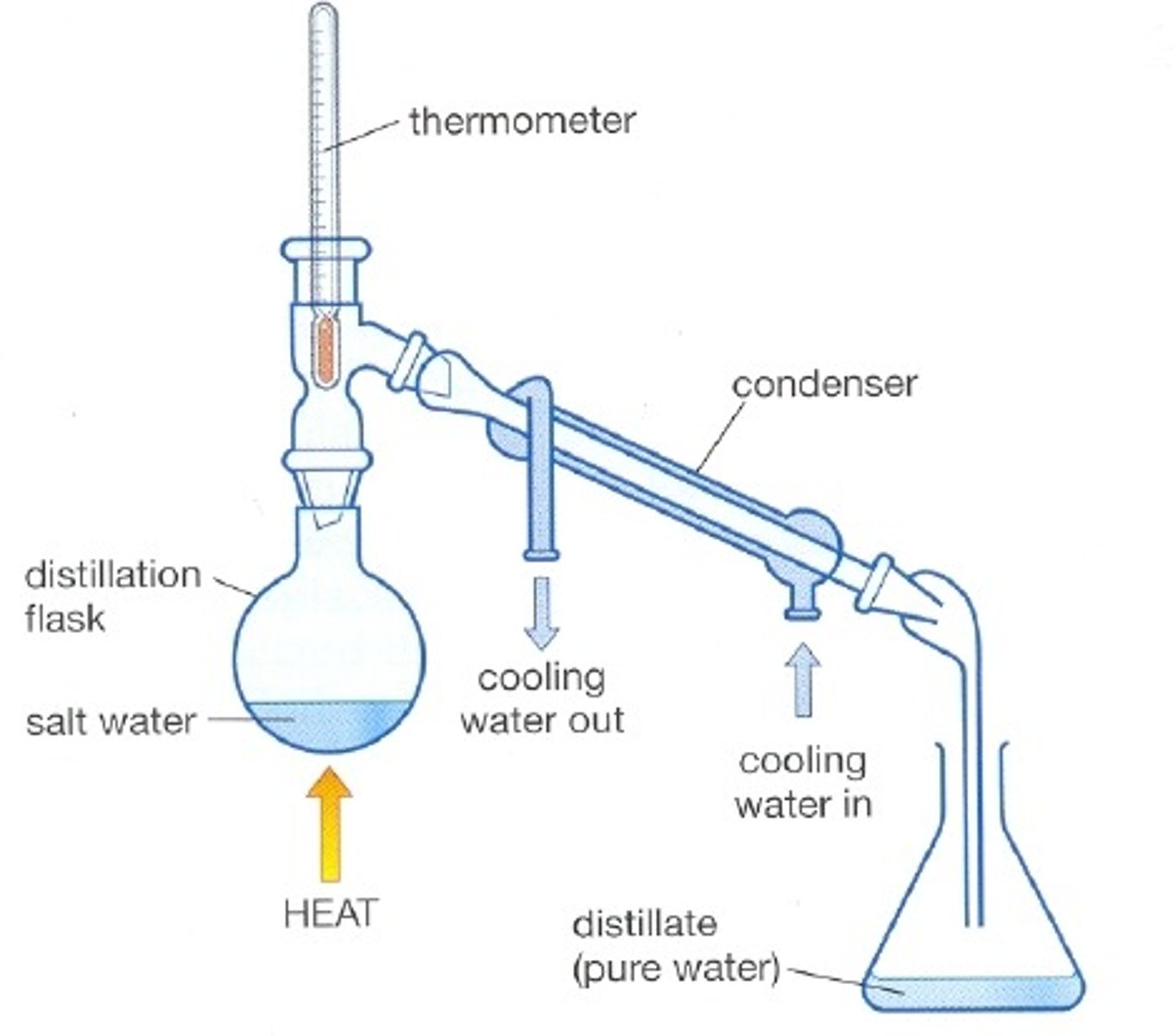
distillation is based on
differences in boiling points
distillation
a liquid is boiled to produce a vapor that is then condensed into a liquid
types of chromatography
thin-layer chromatography (TLC), paper, column, gas, size exclusion, ion, reverse, affinity
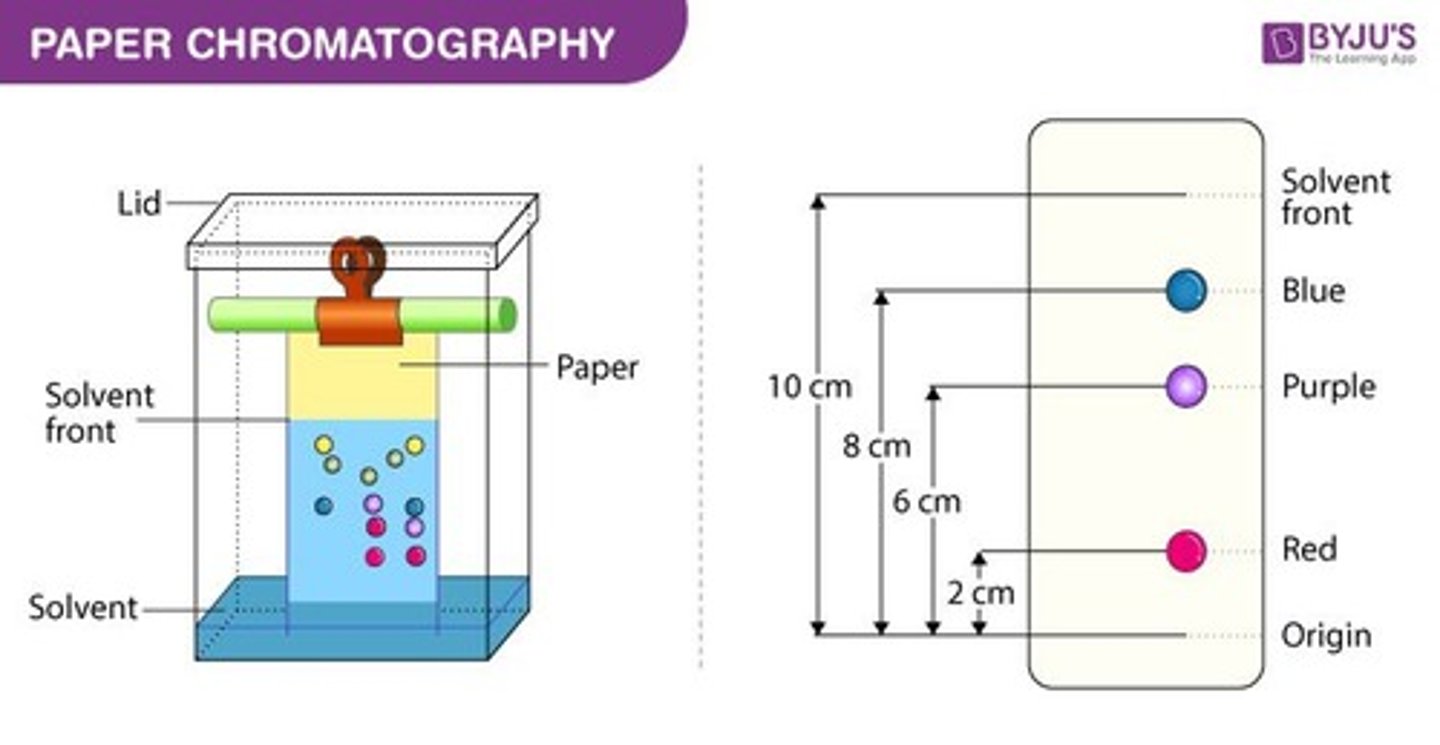
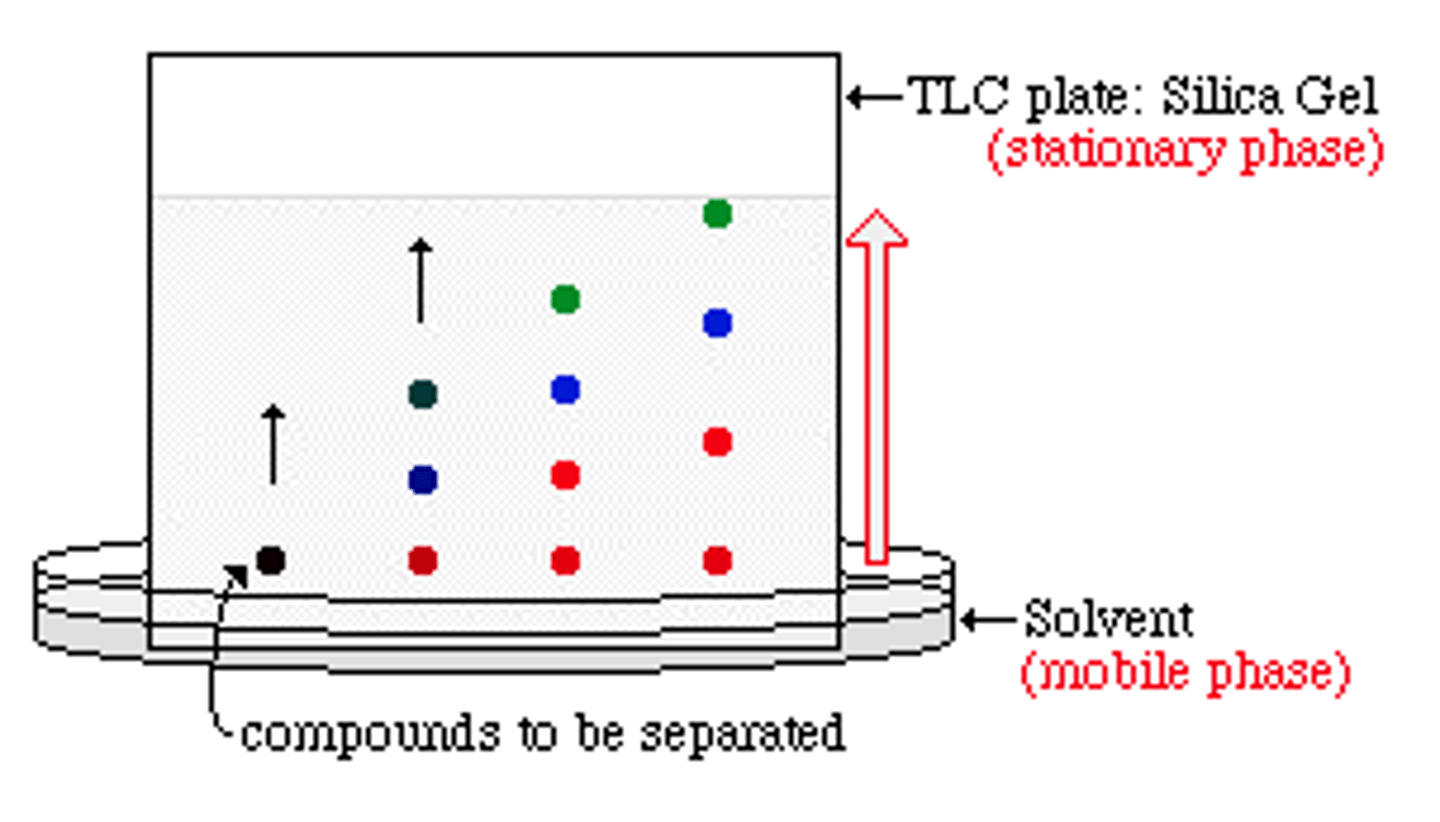
How does chromatography work?
A mixture is separated by being diluted and passed over a matrix that adsorbs different compounds with different affinities based on polarity and solubiility
sublimation in separation
separates two solids when one sublimates and is then condensed and solidified

thin layer chromatography
a separation technique that involves the separation of small molecules as they move through a silica gel and an eluting solution
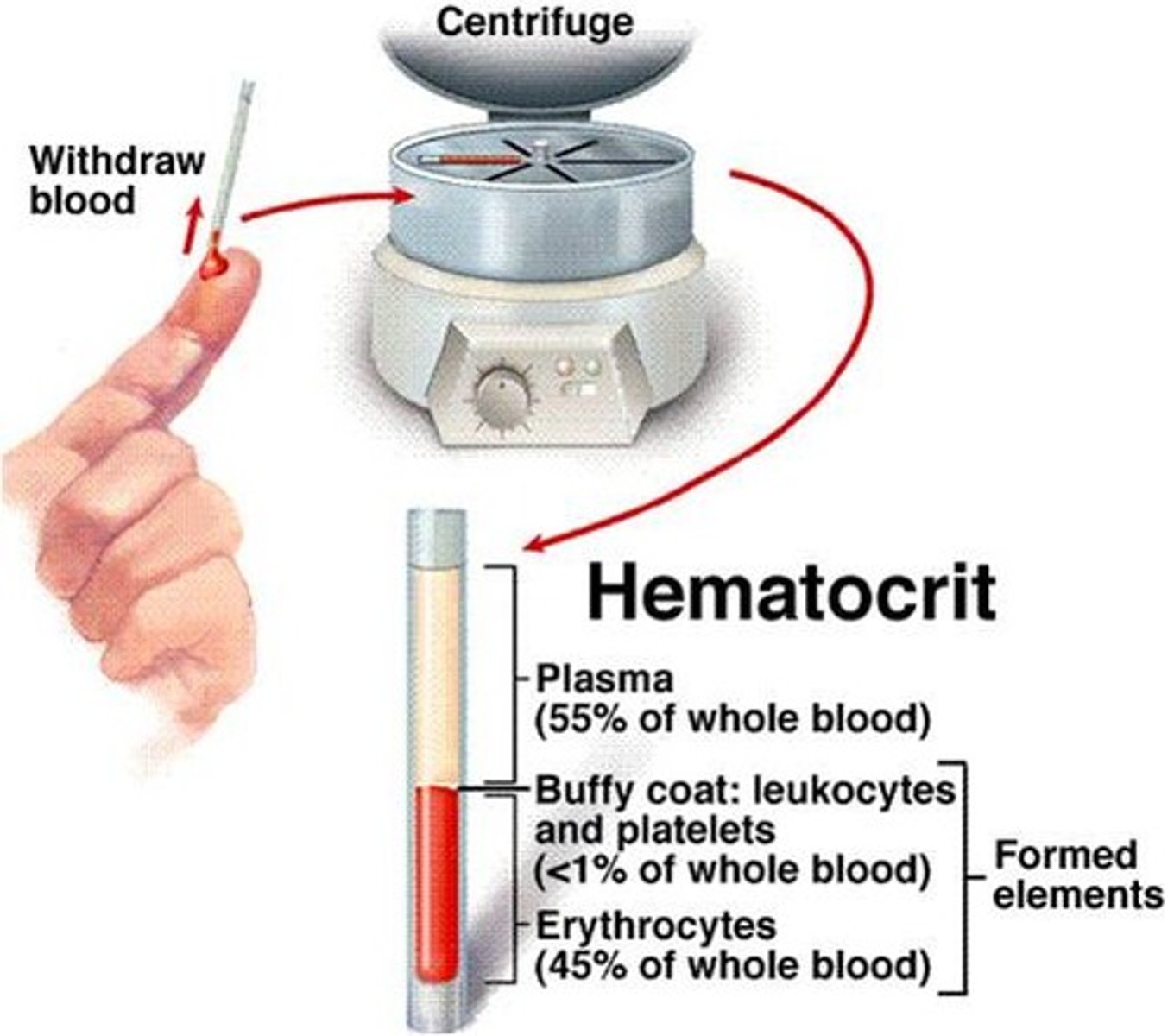
centrifugation
Separates components by density and mass using high-speed spinning
substances used in this experiment (separation)
benzhydrol/benzoic acid, salt (NaCl), and sand
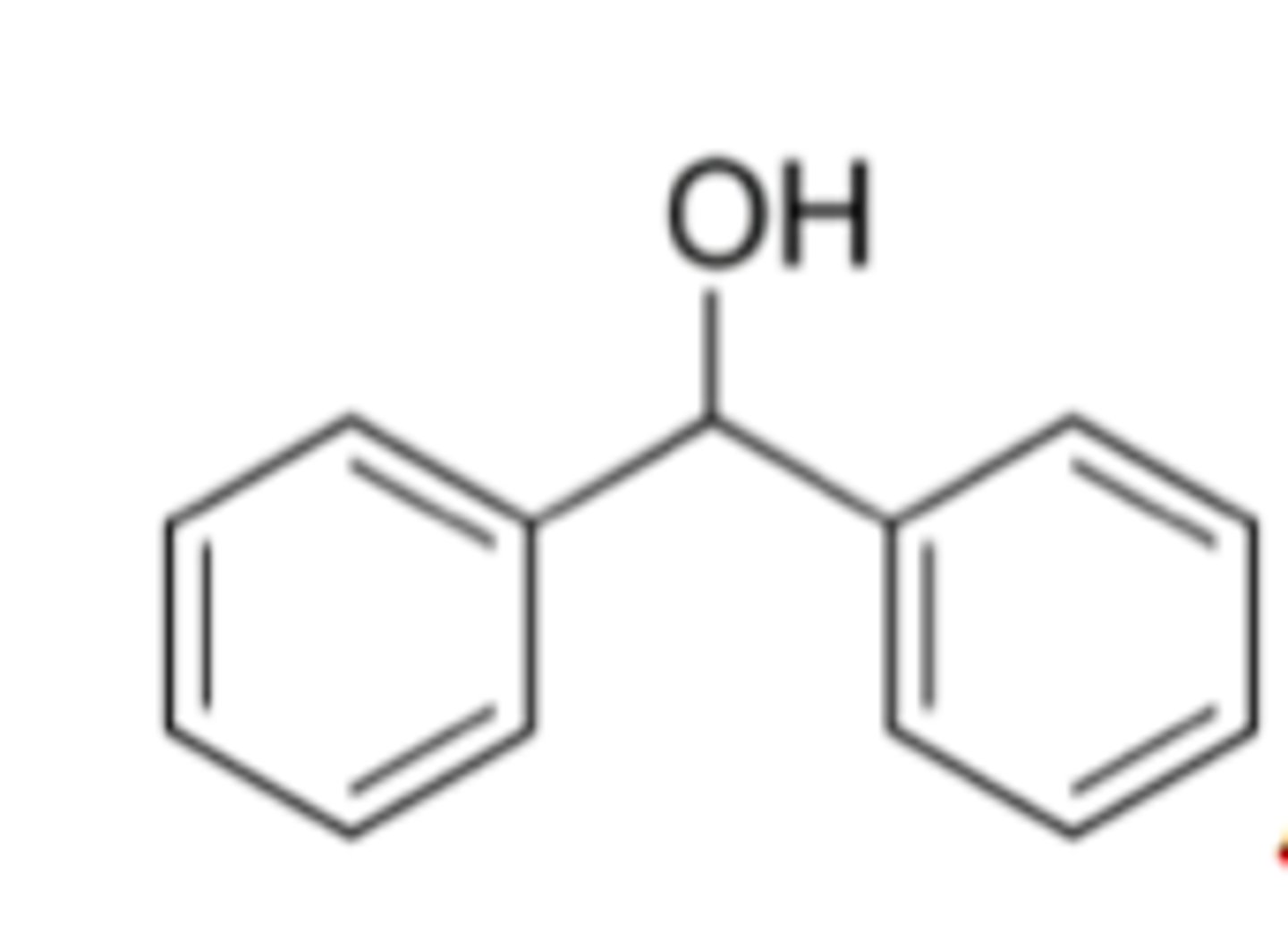
benzhydrol
also known as diphenylmethanol
benzoic acid MP/BP
122C/250C
benzhydrol BP/MP
298C/69C