Chapter 12 Part Two: Chromatin Remodeling
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
what is chromatin remodeling
cahnges nucelosome density or position and is an itnergral part of eukaryotic gene regulation. this repositioning of nucelosomes allows chromsone to be accessible to trasncription regualtory proteins, rna polymerase II and much more.
what is one of the best studied chromatin remodeling compelxes
SWI/SNF
chromatin remodeling exposes _____ sequences. Histone octamer _____ in response to chromatin remodeling activity such as the SWI-SNF complex.
regulatory
slides
what is teh SWI/SNF complex
The SWI/SNF complex uses energy from ATP to act like a tiny, specialized crane or bulldozer that moves those histones. It can:
Slide the DNA spools along the strand.
Shove the spools aside (evicting them).
Change the way the DNA is wrapped around the spool.
By doing this, the SWI/SNF complex opens up or closes down sections of the DNA. This action is crucial because it controls whether Transcription Factors (the proteins that turn genes on or off) can access the DNA to start the process of gene expression.
what does Snf stand for
Sugar nonfermenting mutants, mutagenized yeast cells were screened for cells that could not grow well on sucrose
SWI stand for
switch mutants,
in another screen mutuanzied yeast cells were screed for mutants that were defencctive in siwthcing their mating type
one mutant gene was found to cause both phenotypes, could neither utilize sucrose nor switch mating type and it was known as the
swi2/snf2 locus “switch sniff”
the SNf2-Swi2 protein is part of a large coplex known as teh SWI-SNF complex. this complex bascially
activates transcription by moving nucleosomes that are covering tata sequences. It faciltiates the inding of rna pol II and is a co-activator
what is short term activation in chromatin environemtnt
the cell's way of quickly turning a gene "on" for a limited time in response to an immediate signal, like a hormone, stress, or sudden change in the environment.
the binding of multiple regulatory proteins to muleitple binding sites in an enahncer can catalyze the formation of an .
enhanceosome
enhanceosomes help recruit transcirptional machinery. for example
TFs recreuit the co-activator protien (CBP) which binds to the Tfs and RNA pol II initiating trasncirption.
the human B-interferon enhanceosome encodes
antiviral protein interferon
how is the b-interferon enhacnecoems important in the immune system
makes the Interferon protein, which is a powerful antiviral agent. When a cell gets infected by a virus, it needs to turn this gene on immediately and to a very high level to fight the infection and warn other cells. When a virus atttacks, the team of poteins, spelcsomes, assembles on the DNA. this assmebly ensures that the cell quickly produces massive amounts of antiverial B interferon protein to gight the infection off.
the assembly of transcription factors into an enhancesosome about 100 bp upstream of the _____ and the trancirpoin statt site
TATA box
GCN5 is a _____ _______ that acetylates H4K8 and H3K9
A histone acetyltransferase (HAT)
Can eukaryotic enhancers act great distances upstream or downstream from a. Gene
Yes
To prevent enhancers from interfering with near by genes what regulatory elements evolved?
Insulators. When positioned between an enhancer and promoter the enhancer blocking insultoaers prevent the enhancers from activating transcription at that promoter
In chromosomes groups of genes are kept apart by _______ formed by interaction of insulator proteins such as CTCF
TADS— topically associating domains
Does chromatin remodeling use energy?
Yes it uses energy produced in ATP hydrolysis to slide, eject or replace histone octamers or exchange variant histone for canonical histone
Molecular mechanisms that alter chromatin structure will
Permit or prevent transcription regulatory proteins access to DNA
What are the three histone tail modifications
1- acetylation
2- methylation
3- phosphorylation
What is long term inactivation of genes in chromatin environment?
Most genes in eukaryotic genomes are off any one time.
What is the difference between human B interferon gene ?
It can be turned on and off in a matter of hours (short term activation)
What is epigentic inheritance
Affects traits of daughter cells without altering DNA sequence. Epigenetic marks will be inherited by the daughter cells then passed down
What are three examples of epigenetic control of transcription?
Position effect variegation
Genomic imprinting
X-Chromosome inactivation
Is chromatin structure inherited from one cell generation to another?
Yes for example, if there’s methylation in the mother cell, then there will be methylation in the daughter cell
Can histone variants be used to rapidly change chromatin in a replication independent pathway
Yes
What is the position effect variegation?
Chromosomal neighborhoods exist that can silence genes that are expiermentally relocated to adjacent regions of chromosome (can silent gene—example PEV in Drophila flies)
How did they discover position effect variegation in drosophila flies
Cytological exmaination revealed that a chromosomal rearrangement called an inversion had taken place in the mutant flies.
Can the spreading of hetreochromating silence genes ? What do you see?
Yes heterochromatin can spread to the neighboring euchoramtin and silence the white gene. When the heterochromatin spreads, there is a mixture of red and white cells compared to the previous all red eye in the wild type.
If you see white tissue: descendent of a single cell has been silenced and remain silence, if you see red patches the heterochromatin has not spread to the white gene, the WT gene remains active.
What are 2 features of epigenetic regulation
Expression of a gene can be repressed by virtue of its position in chromosome rather than by mutation in its DNA sequence
Epigenetic silencing can be inherited from one cell generation to the next
What does the Su(var) gene do
It is a suppressor that when mutated will reduce the spread of heterochromatin. It has wild type products which are required for heterochromatin spreading.
What is the Su(var) protein that is associated with heterochromatin formation and is associated with heterochromatin telomere and centromeres
HP1 gene.
When Su(Var) is mutated what is the color of the eyes?
When enhancer variegation Evar is mutated what happens with the eyes>
So when suvar is mutated, there is a decrease in heterochromatin spread which leads to the red eyes.
When the evar is mutated, it increases the spread of heterochromatin and makes more cells with the silenced white+
Why does HP1 protein bind to some DNA regions and not to others
HP1 only binds where there’s been methylation
HP 1 will call for more HMTase which will
Add more methyl groups to specific amino acid residue in lysine 9. HP1 molecules will bind to each other and cause modified nucleosides to form a MORE CONDENSED chromatin state and heterochromatin spreading
What does H3K9me stand for
Histone 3 Lysine 9 methyl added
Chromatin that contain nucleosomes that are methylated at H3K9me, will
cause more HP1 protein binding and more heterochromatin formation
What are barrier insulators and what do they do
They stop the spread of heterochromatin by creating a local environment that is not favorable to heterochromatin formation.
What can move the barrier insulators so that it no longer prevents heterochromatin spreading
An inversion
What is gender specific silencing of genes?
Genes or chromosomes are silenced in males or females but not both
What are imprinted genes
Controlled by DNA regulatory elements called imprinting control regions that have parent specific chromatin modifications.
What is maternal imprinting
Copy of the gene derived from the mother is inactive. (Allele is only expressed if inherited from father)
What is paternal imprinting?
When the paternal Copy is inactive, meaning that the allele is only expressed if it is inherited by the mother
The imprinting control regions is different in males vs females explain this.
In males the ICR is methylated blocking the CTCF insulator protein from binding and directing the enhancer to active transcription of IPf2
In females the ICR is unmethylated in so CTCF insulator protein CAN BIND and forms an insulator that blocks the enhancer activation of IPf2 and directs activation of H19.
Imprinted genes are expressed as if there were only _____ ______ of gene present in the cell (haploid) even though there are _____ (diploid)
One
Two
Are there changes in DNA sequences of imprinted genes
No
If dna sequence of a gene does not correlate with activity what does
Epigenetic marks such as DNA methylation that cause genes to be expressed in parent origin manner are established in germ cells such as sperma and eggs and as organisms develop are maintained through mitosis cell division of somatic cells.
For autosomal genes, the number of transcripts produced by a gene is ______ to the number of copies of the gene in a cell
Proportional
What is Xist lncRNA.
LncRNA is a long noncoding RNA, that plays a role in initiating the silencing of one of the X chrosmoems
What is dosage compensation?
Makes the amount of most gene products from the two copies of the X chromosome in females equivalent to the single dose of X chromosome in males
X link gene determines color. Assume that X+ produces black hair. X0 produces organogenesis fur, and X+X0 produces tortioshell . How would this affect females vs males
Females could be orange, black or tortorisell
Males could only be orange or black
What is an inactivated chromosomes called
BARR BODY
X inactivation was discovered by who
Mary F Lyon
What exactly is X inactivation ? How does it work?
Aka lionization, it is a genetic process in female mammals that ensures dosage compensation for X linked genes.
early in female embryonic development one of the two X chromosomes is randomly chosen to be silenced. The chosen inactive X undergoes structural changes and is heavily condensed to form a BARR body.
Epigenetic modification is made, and once the X chromosome is inactive in the embryonic cell it would remain inactive in all the cells that descend
What is the BARR BODY
Inactive highly condensed X chrosmone found in the somatic cells of female mammals
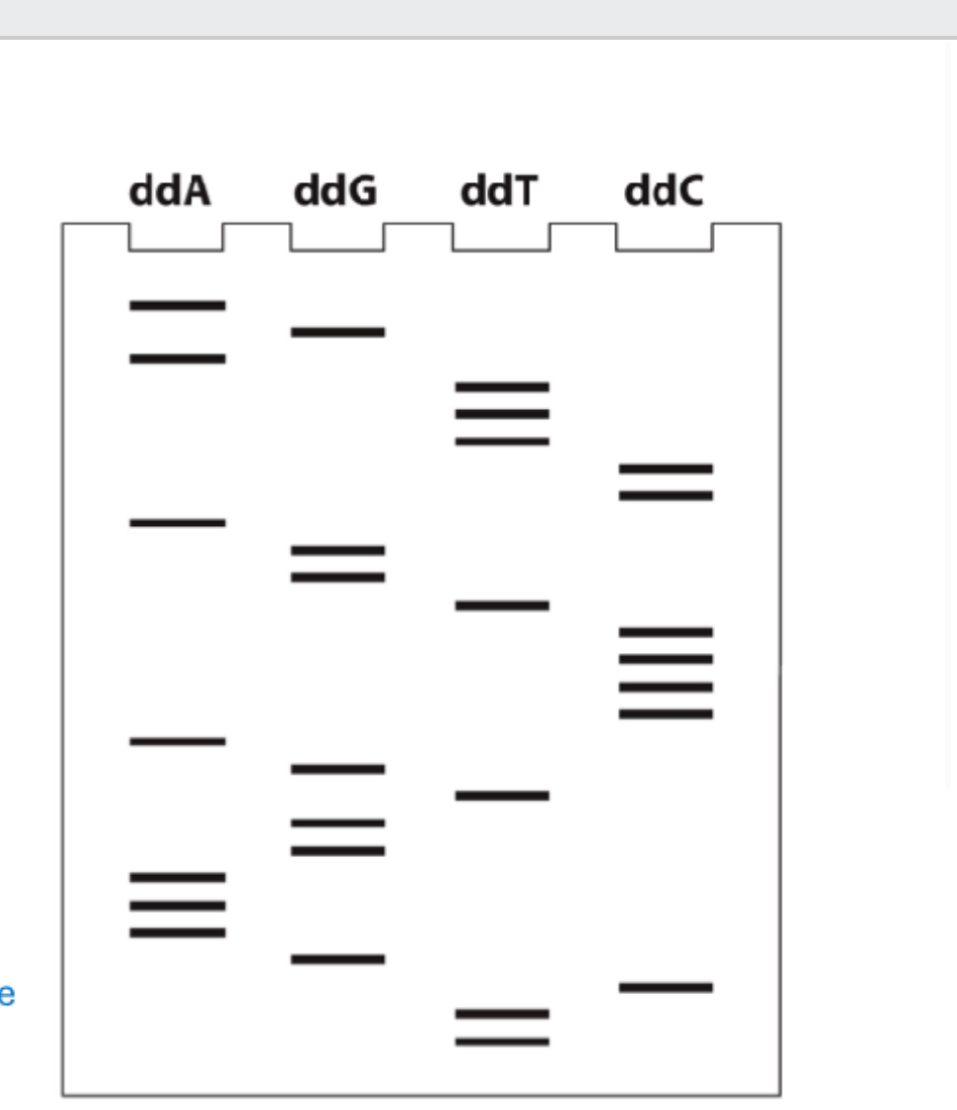
Label the nucleotide sequence synthesized from primer. Also label nucleotide sequence used as template strand.
5’ TTCGAAAGGTGACCCCTGGACCTTTAGA3’
3’ AAGCTTTCCACTGGGGACCTGGAAATCT5’