Acids, Bases and Buffers
1/28
Earn XP
Description and Tags
From lectures 7 onwards
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
State the Bronsted-Lowry definition for acids and bases
Bronsted-Lowry definition, An acid is a substance that is a proton (H+) donor. A base is a substance that accepts a proton (H+).
State the Lewis definition for acids and bases
Lewis definition, An acid that is a substance which is an electron pair acceptor. A base is a substance which donates an electron pair.
State the definition for conjugate acids and bases
The conjugate base, is the species formed after an acid has lost H+
The conjugate acid, is the species formed after a base gains H+
State the equilibrium and its constant for a general acid ,HA, in water
The equilibrium constant is written in terms of concentration so it is Kc

When do we use Ka rather then Kc
We use Ka for dilute solutions as the concentration of H2O will remain essentially constant, so we can use the acid dissociation constant, Ka, instead of Kc
What’s the pKa equation and why do we usually use it rather then Ka
As acidity can vary over such large range it is more usual to consider acid strength in terms of pKa
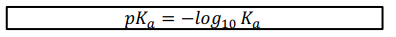
Relation between Ka and pKa for strong and weak acids
-Strong acids have high values of Ka, and low values of pKa
-Weak acids have low values of Ka, and high values of pKa
What dictates acidity (4 Factors)
The strength of the H-A bond
The electronegativity of A
The stabilization of A- compared to HA
The solvent (Levelling effect)
What does a polybasic acid refer to
Refers to an acid that can loose more than one proton per molecule
Describe relationship between mesomeric effects and acidity
Mesomeric effects refer to a type of resonance where the substituent group (Negatively charged atom) spreads charge across a molecule.
Depending on if its a M+ or M- group it will destabilize or stabilize the negative charge of the molecule subsequently decreasing or increasing acidity
Describe how mesomeric effects can increase acidity
Requires an M- group (such as NO2)
The negative charge will be delocalised through resonance all the way to the M- group
This will place the negative charge on the most electronegative elements, stabilising the conjugate base
Having a more stabile conjugate base means an acid is more likely to donate a proton making it more acidic
Briefly ;
Adding electron accepting groups results in spreading the negative charge out, and relocating it to electronegative atoms will stabilise the conjugate base and decrease pKa, making it more acidic.
Describe how mesomeric effects can decrease acidity
Requires and M+ group (such as NH2)
The M+ group will increase the electron density decreasing the stability of the anion, as it is trying to donate its lone pair
This destabilises the negative charge resulting in a less stable conjugate base
Having a less stable conjugate base means an acid is less likely to donate a proton making it less acidic
Briefly;
Adding electron donating groups results in adding more electrons to an already electron rich system, destabilising the negative charge resulting in increasing pKa, making it less acidic
Why is it hard to differ between very strong acids when dissociated in water
The very strongest acids appear to have the same strength as they are completely dissociated.
This is because water is quite a good proton acceptor and therefore all strong acids appear to be the same.
How to solve the issue of waters solvent levelling when attempting to differ between strong acids
To differentiate very strong acids, use a solvent that is more difficult to protonate than water, for example ethanoic acid, propanation of ethanoic acid is more difficult then water allowing us to differentiate between HCl and H3O+
Describe how solving the issue of waters solvent levelling when attempting to differ between strong bases works on the same principle as strong acids
Any base appreciably stronger than water will deprotonate it to give OH– (aq) so the solution behaves as though it is composed of OH– (aq) ions. As such we use a solvent such as liquid ammonia which wont deprotonate as easily
give the Kb
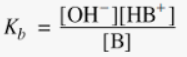
give the pKb equation
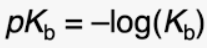
How do we usually describe the strength of a base rather the Kb
In most cases, chemists actually describe the strength of a base in terms of the pKa of the conjugate acid
The stronger the base, the weaker the conjugate acid, therefore lower Ka and higher pKa
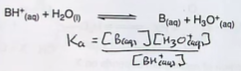
How are buffer solutions most commonly made
Buffer solutions are normally made from weak acids and their conjugate bases or from weak bases and their conjugate acids
How do buffers resist change in pH from strong acids and bases
(Using a mixture of CH3COOH and CH3COONa as an example)
Using a mixture of CH3COOH and CH3COONa as an example,
When we add a strong acid, due to the amount of Ch3Co2- (conjugate base) the acid will react with it instantly and covert into ethanoic acid. Converts the strong acid into a weak acid
When we add a strong base, This will react with H3O+ (conjugate acid), this will dissolve in the lower equilibrium moving to compensate for its loss. This will counter any change in pH
What determines buffer capacity
Primarily on the concentrations of salt and acid used.
What is the Henderson-Hasselbalch equation
The pH of a buffer solution will normally be close to the pKa of the acid being used and can be tuned by varying A/HA
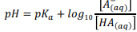
Give the equilibrium equation for the self-ionisation of water into cations and anions
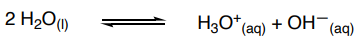
Give the Kw equation
As H2O is effectively constant in the dissociation of water we can write a new expression being Kw
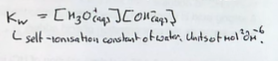
Why does pH of water vary with temperature
Dissociation of water is endothermic, hence equilibrium moves to the right as temp increases.
Means that the pH of water varies with temperature.
Why do we need to use pKa to determine [H3O]
Weak acids are not fully dissociated and therefore [𝐻3𝑂] ≠ acid added. Therefore we need to use the pKa to determine [𝐻3𝑂] .
What is degree of dissociation
Defined as the ratio between the amount of dissociation compared to the starting concentration expressed as a percentage.
How do we determine degree of dissociation
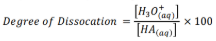
How to estimate the concentration of an acid or a base in solution using titration. (5 points)
Use of an indicator or using a pH meter to determine the end-point
Recall that the change in pH is not linear with the amount of acid (or base) added.
Plot the change in pH as a function of added acid
End up with a titration curve
Titration curves depend on whether the acid and/or the base are strong