Cholesterol synthesis + reverse cholesterol pathway + cholesterol information
1/21
Earn XP
Description and Tags
structures and steps
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
synthesis of cholesterol
All of its carbon atoms are provided by acetate (activated form is acetyl-CoA)
Isoprene units are essential intermediates in the pathway
four stages of cholesterol synthesis from acetyl-CoA quick summary
Condensation of three acetate units to form mevalonate
Conversion of mevalonate to activated isoprene units
Polymerization of six isoprene units to form the 30-carbon linear squalene
Cyclization of squalene to form the four rings of the steroid nucleus
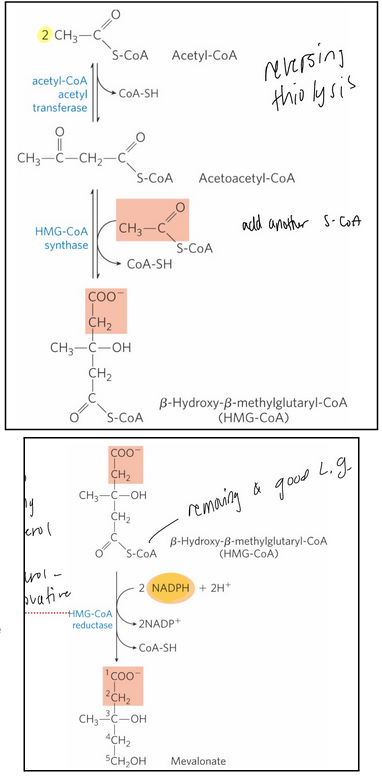
Stage 1 of cholesterol synthesis—> 3 steps in detail (including 3 enzymes and structures)
synthesis of mevalonate from acetate
acetyl-CoA acetyl transferase = catalyzes the condensation of two acetyl-CoA molecules
HMG-CoA synthase = catalyzes the condensation of acetyl-CoA with acetoacetyl-CoA to form 𝛽-hydroxy-𝛽-methylglutaryl-CoA (HMG-CoA)
HMG-CoA reductase = an integral membrane protein of the smooth ER that catalyzes the reduction of HMG-CoA to mevalonate
The major point of regulation on the pathway to cholesterol
Catalyzes the committed step
Requires 2 molecules of NADPH
Enzyme is inhibited by statin
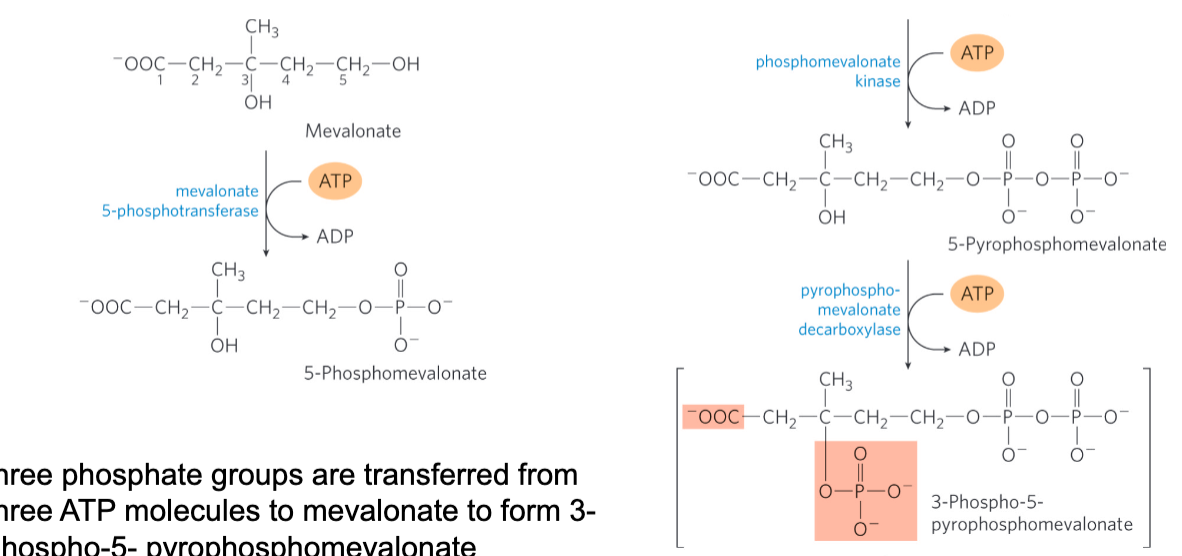
stage 2 of cholesterol of synthesis
Conversion of mevalonate to two activated isoprenes
1 phosphate is not sufficient
Final product has a good leaving group, CO2 and one phosphate on its beta carbon and the other two on the 1st carbon
Three phosphate groups are transferred from three ATP molecules to mevalonate to form 3-phospho-5-pyrophosphomevalonate
CO2 and Pi leave to produce a double bond in ∆3- isopentenyl pyrophosphate
The first activated isoprene
Isomerization of ∆3-isopentenyl pyrophosphate yields dimethylallyl pyrophosphate
The second activated isoprene
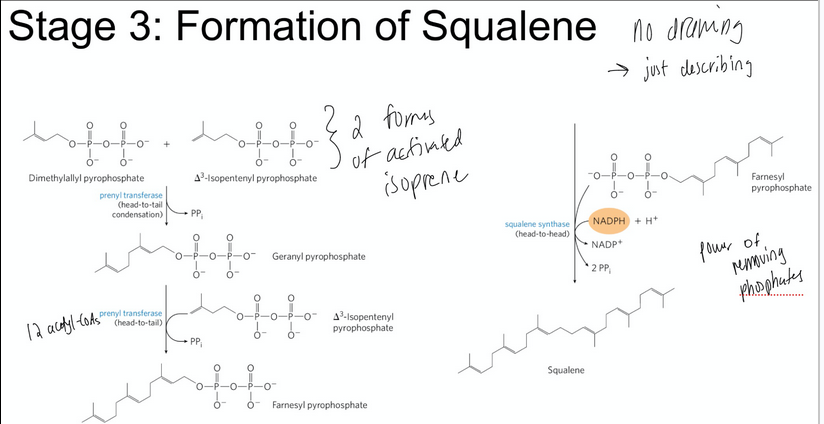
stage 3 of cholesterol of synthesis
condensation of six activated ispoprene units to form squalene
The two activated isoprenes undergo head-to-tail condensation to form geranyl pyrophosphate
Geranyl pyrophosphate undergoes head-to-tail condensation with isopentenyl pyrophosphate to form farnesyl pyrophosphate
Two molecules of farnesyl pyrophosphate join head-to-head to form squalene
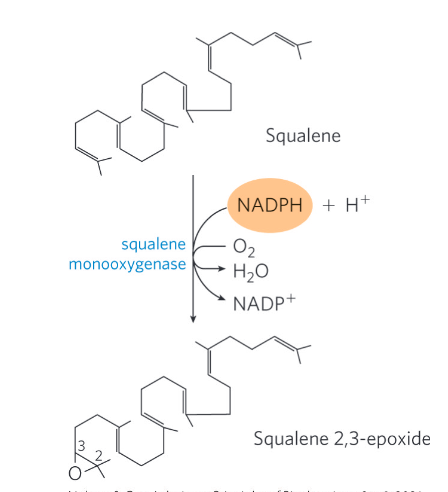
stage 4 of cholesterol of synthesis
Conversion of squalene to the four-ring steroid nucleus
Squalene monoocygenase = adds one oxygen atom from O2 to the end of the squalene chain to form squalene 2,3-epoxide
Mixed-function oxidase
Requires NADPH
Cyclization of squalene 2,3-epoxide forms lanosterol
A series of approx. 20 reactions converts lanosterol to cholesterol
In plants, the epoxide cyclizes to other sterols, such as ergosterol
cholesterol has several fates
Cholesterol is synthesized primarily in the liver
Most of it is exported as:
Bile acids
Biliary cholesterol
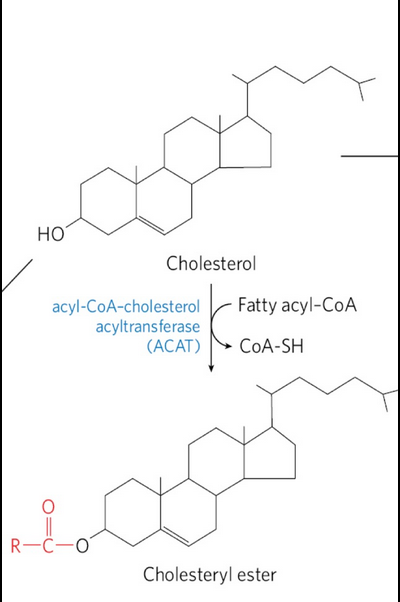
Cholesteryl esters
Formed in the liver by acyl-CoA-cholesterol acyltransferase (ACAT)
export of cholesterol as bile acids
Bile acids= the principal components of bile, a fluid stored in the gallbladder
Excreted into the small intestine to aid in the digestion
Relatively hydrophilic cholesterol derivatives that serve as emulsifiers
Made from cholesterol
Ex. taurocholic acid
export of cholesterol as choelsteryl esters
Cholestereyl esters = cholesterol molecule with a fatty acid from coenzyme A attached to its hydroxyl group
Formed in the liver by acyl-CoA-cholesterol acyltransferase (ACAT)
Cannot function appropriately in membranes due to high hydrophobicity
Transported to other tissues or stored in the liver in lipid droplets
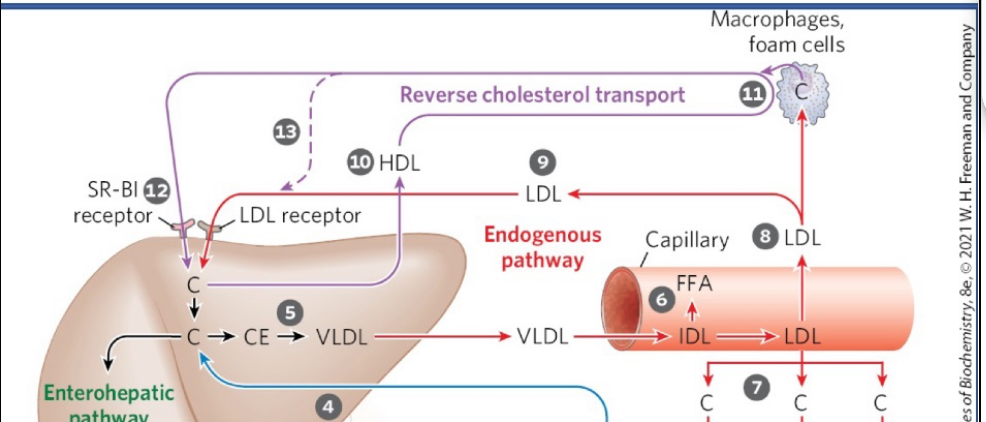
the reverse cholesterol pathway
HDL acts as the main carrier in this pathway, collecting excess cholesterol from cholesterol-rich extrahepatic tissues and transporting it back to the liver.
In the liver, cholesterol can be:
Converted to bile salts and stored in the gallbladder, or
Excreted into bile for elimination.
Macrophages in arterial walls can engulf LDL particles; the cholesterol esters are hydrolyzed to free cholesterol. If excess cholesterol accumulates, it can crystallize. When macrophages rupture, this contributes to plaque formation (atherogenesis).
The SR-BI (scavenger receptor class B type I) receptor on hepatocytes binds HDL and mediates selective uptake of cholesterol, allowing HDL to unload its cargo without being fully degraded.
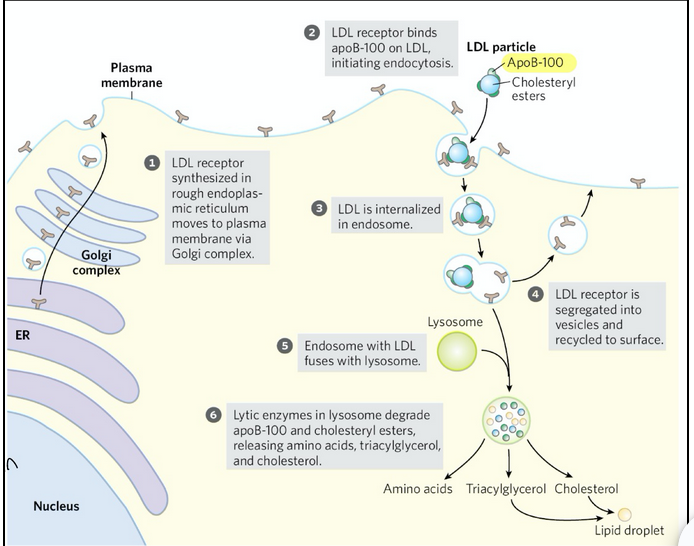
steps of receptor-mediated endocytosis for cholesterol esters entering cells
LDL receptors are made in the rough ER and trafficked to the plasma membrane through the Golgi.
LDL receptors on the cell surface recognize and bind apoB-100 on LDL particles
The receptor-LDL complex is brought into the cell via endocytosis
The receptor is recycled back to the plasma membrane so it can capture more LDL
The endosome with LDL fuses with a lysosome
Lysosomal enzymes degrade apoB-100 and cholesteryl esters → releasing free cholesterol, amino acids, and triacylglycerols
This is the mechanism of LDL uptake into cells, which is part of the cholesterol delivery side of the cycle. Reverse cholesterol transport (HDL pathway) is the protective counterbalance that prevents overload of cholesterol in tissues by bringing it back to the liver
PCSK 9
PCSK 9 protein degrades LDL receptors so if PCSK9 isn’t inhibited, fewer receptors return to the membrane → higher LDL levels in blood
Low-density lipoprotein and LDL receptors def
Low-density lipoprotein (LDL) = formed by triacylglycerol loss in VLDL
Rich in cholesterol and choelsteryl esters
Carries cholesterol to extrahepatic tissues and macrophages
LDL receptors = receptors in the hepatocyte plasma membrane that take up LDL not taken up by peripheral tissues and cells
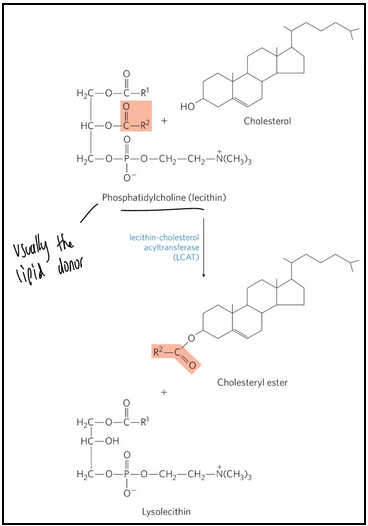
HDL def and role in formation of cholesteryl esters
High-density lipoprotein (HDL) = lipoproteins that originate in the liver and small intestine as small, protein-rich particles
Contain lecithin-cholesterol acyltransferase (LCAT) to catalyze the formation of cholesteryl esters
Mediates cholesterol scavenging and transport back to the liver; ie. carries out reverse cholesterol transport
dysregulation of cholesterol metabolism can lead to…
cardiovascular disease
Atherosclerosis = the obstruction of blood vessels from the pathological accumulation of cholesterol (plaques)
Foam cells = form from macrophages
Macrophages stuffed with cholesterol → foam cells
Shatters immune cell leads to cholesterol plaque build up in arteries
Upregulating HDL doesn’t work to regulate cholesterol/heart disease
It is better to prevent synthesis of cholesterol
statins
Drug class used to treat patients with elevated serum cholesterol
Resemble mevalonate
Are competitive inhibitors of HMG-CoA reductase
By blocking this step, all downstream steps in cholesterol biosynthesis are halted (since mevalonate is the precursor for cholesterol and other isoprenoids)
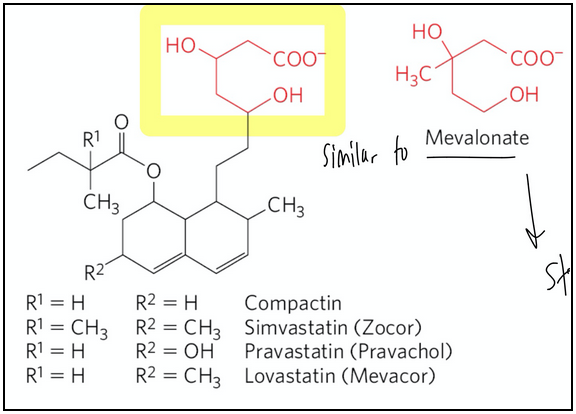
why is HMG-CoA reductase a good drug target for restricting cholesterol synthesis?
It is the commitment step in cholesterol synthesis
Familial hypercholesterolemia
genetic linked disorder
Characterized by extremely high blood levels of cholesterol
Due to defective LDL receptor (causing rapid accumulation of LDL particles)
Cholesterol accumulates in foam cells and contributes to the formation of artherosclerotic plaques
why does reverse cholesterol counter plaque formation and atherosclerosis?
Since HDL removes cholesterol from peripheral tissues and carries it to liver
familial HDL deficiency and Tangier disease
Familial HDL deficiency = HDL levels are very low (very high cholesterol guaranteed)
Tangier disease = HDL levels are almost undetectable
Both are a result of mutations in the ABCA1 protein
ABCA1 protein transports cholesterol into the HDL
apoA-I in cholesterol-depleted HDL cannot take up cholesterol from cells that lack ABCA1 protein
HDL cannot mature → HDL levels in plasma are very low or absent
Cholesterol builds up inside cells, especially macrophages and tissues
True or False —> A diet low in cholesterol would make a meaningful difference in cholesterol build up in individuals with Tangier disorder, as they have a mutation/variant in a gene that is involved in cholesterol trafficking
FALSE since most cholesterol is synthesized rather than consumed so would need pharmacological treatment
elevated cholesterol and TAGs increases ____
LDL
Decreasing LDLs or increasing LDL uptake (recycling) back into the liver is protective against heart disease