Chp 4 - Protein Structures
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
Draw out the types of bonds and their qualities
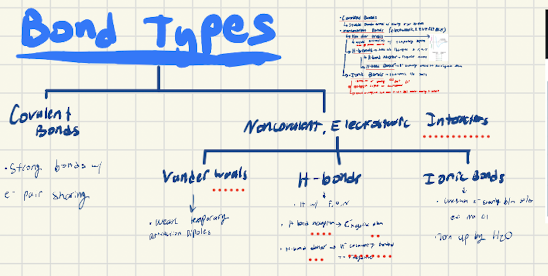
What are the 4 main protein structures, what parts of the amino acid are involved?, and what bonds stabliilze each of them?
==

How are Peptide Bonds formed?
Dehydration rxn (H2O produced as a side product)
What is the pKa for a-COOH & a-NH3 WHEN ISNIDE OF A PROTEIN SEQUENCE?
a-COOH → pKa = 3
a-NH3 → pKa = 9
Do mutations have affects?
Yes. Even mutation in one amino acid → can mess up whole protein
What are the two types of secondary structures and what are they connected/stabilized by THE MOST"?
a-helices
B-sheets
Both stabilized by H-bonds
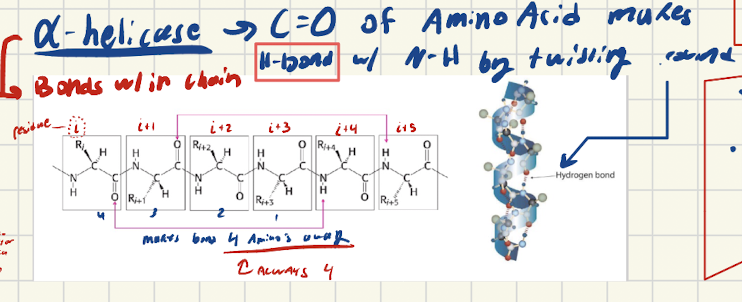
What are a-helives? How are a-helices stabilized?
Bonds WITHIN same chain
C=O makes H-bond with a-amino (NH) that is 4 AMINO ACIDS away from it
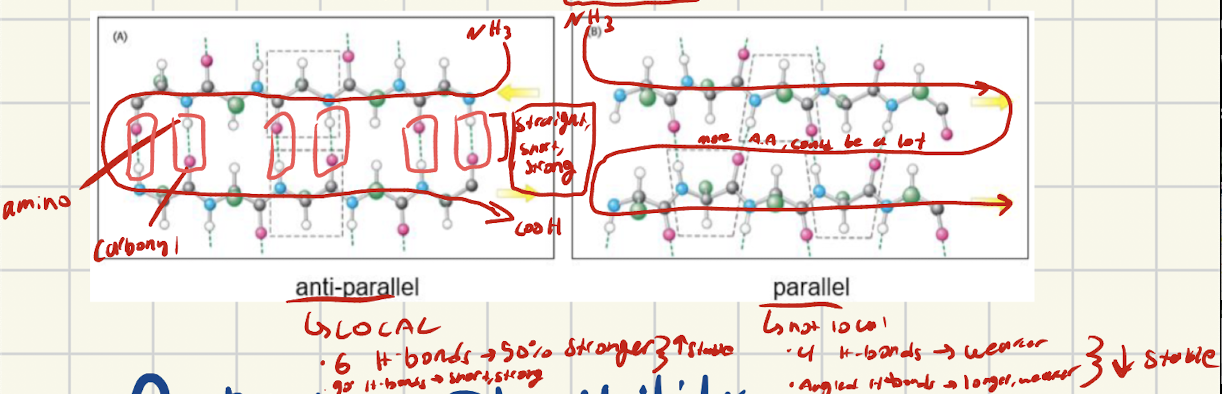
What are B-sheets? How are B-sheest stabilized? What are the different types?
Bonds BETWEEN DIFFERENT chains
Parallel, antiparallel, mixed
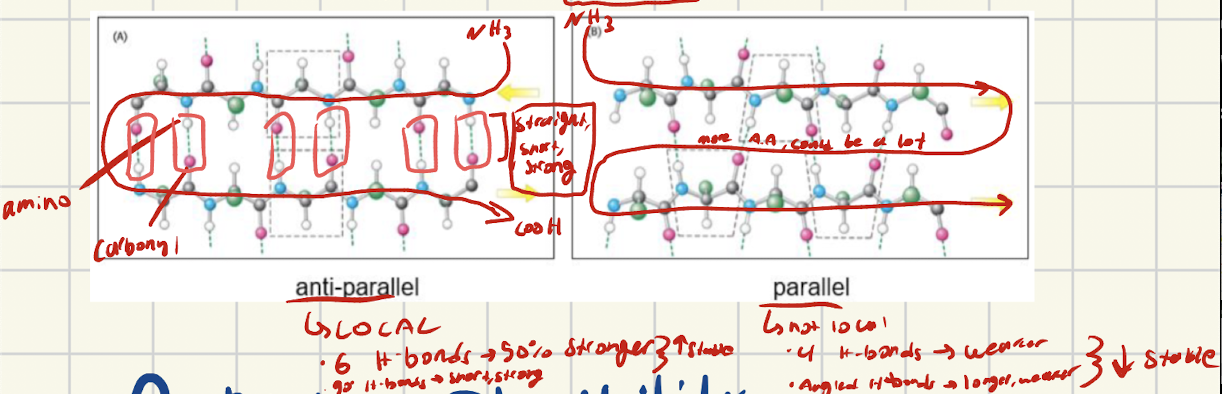
What are antiparallel chains, how are they set up? ]
[LOCAL] The two chains are lined up in opposite directions
One chain has NH3 on the left to start
The other chain has COOh on the left to start
![<p>[LOCAL] The two chains are lined up in opposite directions</p><ul><li><p>One chain has NH3 on the left to start</p></li><li><p>The other chain has COOh on the left to start</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/3f3e17f2-77a4-4822-9c49-255bb47f204b.png)
What interactions do antiparallel bave and why are they stronger than parallel
Antiparallel alignment creates straight, short, strong hydroge bonds. Also more H-bonds form, making antiparallel 50% stronger than parallel.
What are parallel chains, how are they set up?
Two chains lined up in same direction
Both start with NH3 (amino on same side) and run parallel to each other
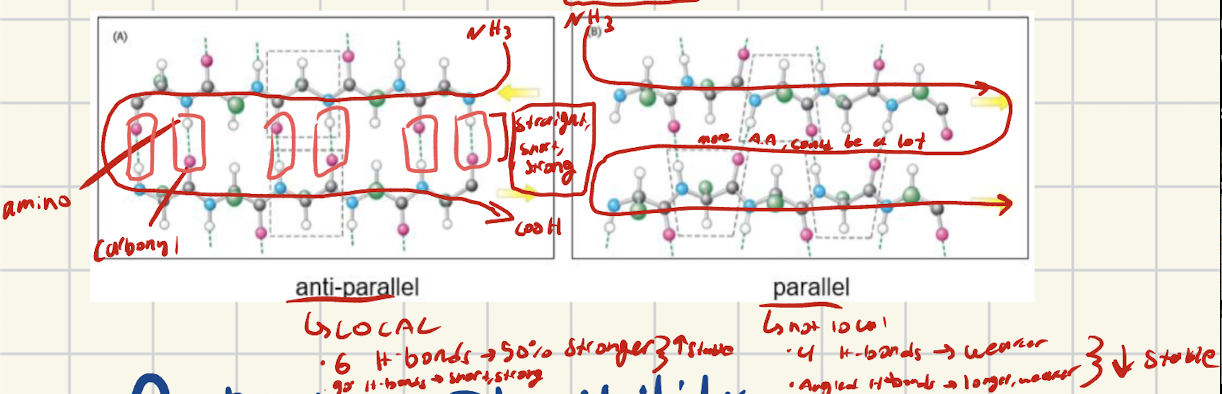
What interactions do parallel and why are they weaker than antiparallel?
Parallel alignment creates long, angled, weak H-bonds. Less H-bonds form bc of alignment
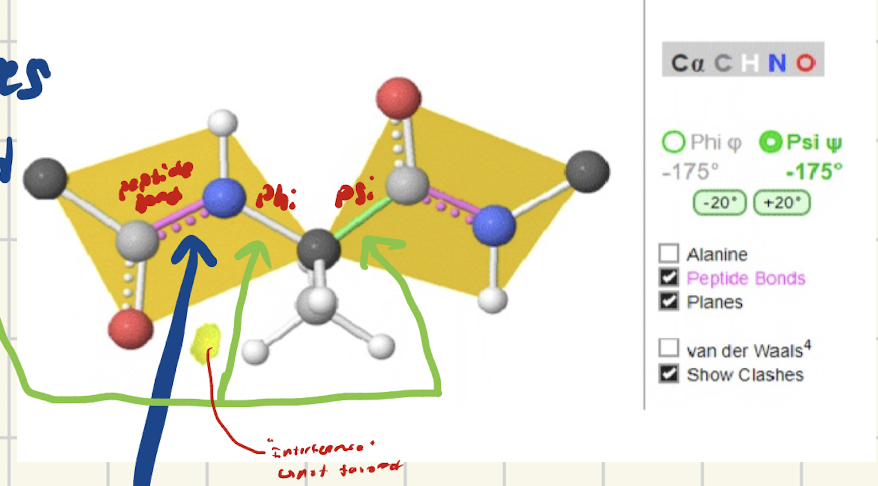
Which bonds rotate in a peptide? which don’t? Why do the bonds want to rotate?
Peptide bonds → NO rotation
Phi bonds → Rotate
Psi bonds → Rotate
Bonds want to rotate to have a more stable conformation
What is a phi bond? What’s it between?
bond that rotates that is between a-amino and a-carbon
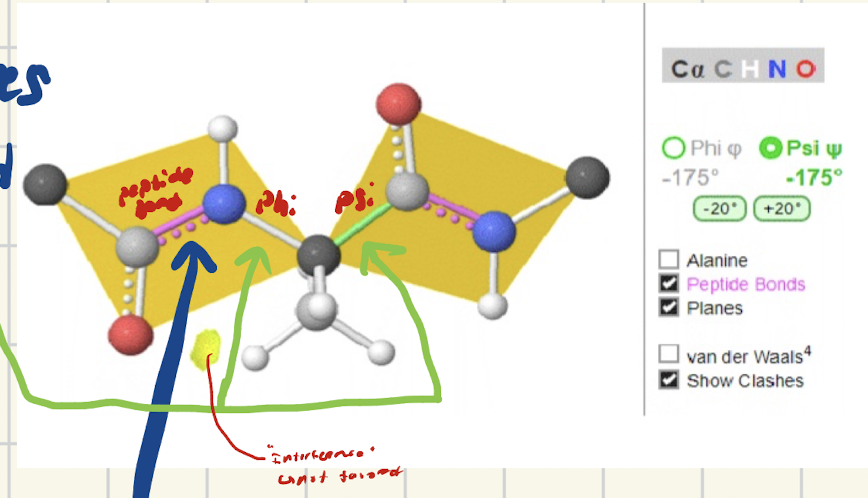
What is a psi bond? What’s it between?
Bond that roates that is between carbonyl carbon (C-C=O) and alpha carbon
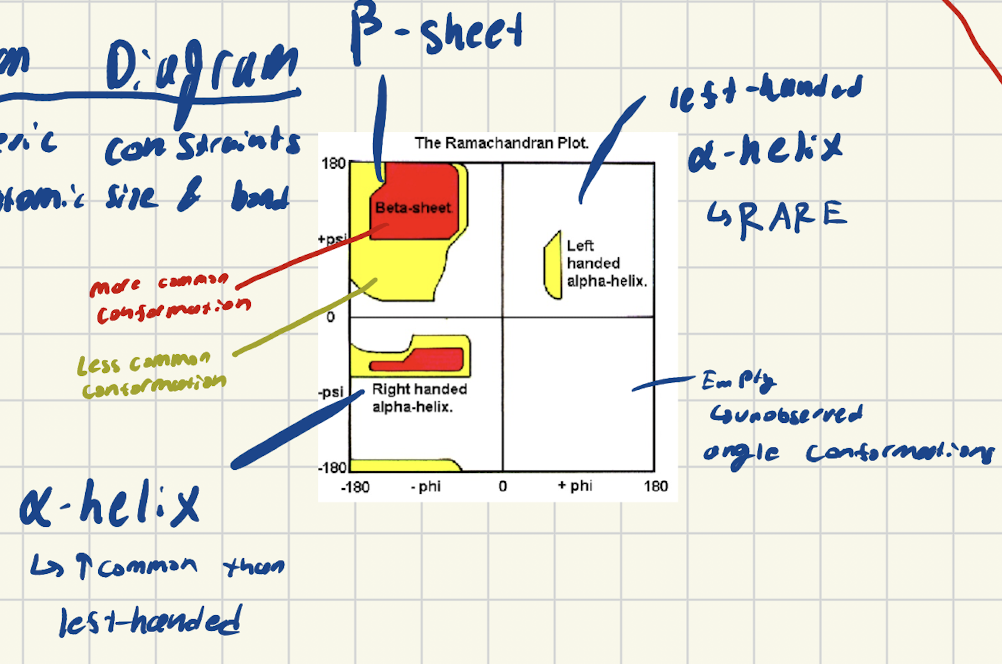
What does the Ramachandram Diagram represent?
Shows steric constraints from atomic size and bond angles for peptides.
illustartes which conformations are the most common
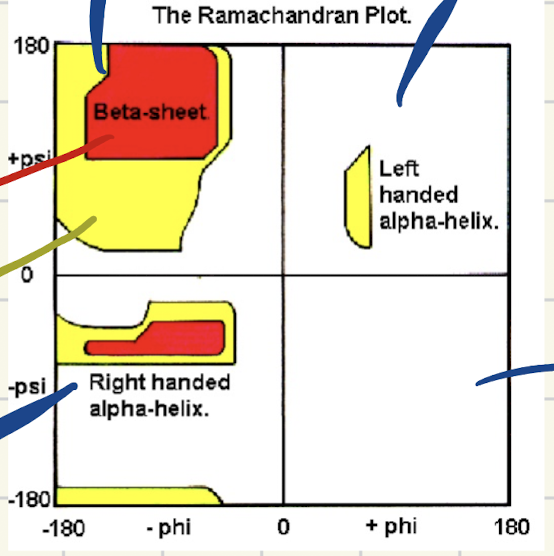
What do the blobs and colors of the rachman diagram saying
What interactions are stabilizing tertiary strcuturs? Which bond type stabilizes the most?
3d structures stabilized w/ mainly electrostaic + Disulfide bonds (IF CYSTEINE IS PRESENT)
Van der Wahls
Ionic Interactions
H-bonds
Disulfide bonds (Covalent0
What is the order of structure for tertiary structures with disulfide bond stabilization w/ electrostatic stabilization. What is the anaology?
TERTIARY Structure
Domains
Motifs
Apartment → Tertiarty structrure
Domain → Rooms within the apartment for their own functions
Motifs → Essential furniture in each specific room for it’s own specific function (ex: oven in kitchen)
WHat are domains? Are they stable independently?
Independent folded stable units within a protein that have their own specific functions
CAN fold stably on own
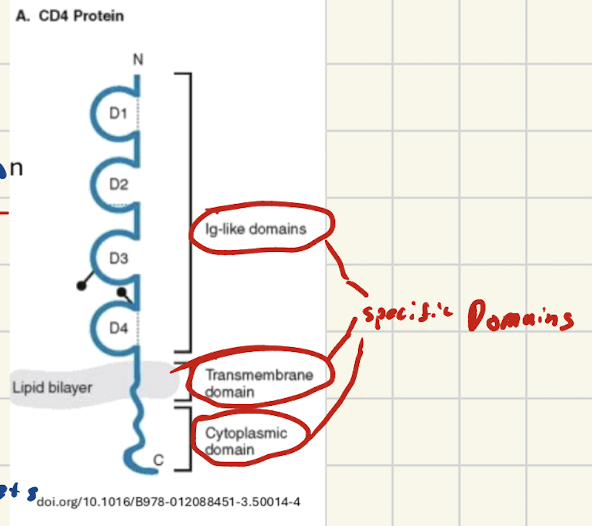
What are motifs? Are they stable independently?
The small recurring patterns (made from a-helices and B-sheets) WITHIN a domain that have their own small specific functions
NOT stable on their own
ARe domains passed throughout evolution
Yes organisms have same domains and have developed and stuck with the same ones
What are examples of Domains that have passed evolutionarly
ATP Binding Domains
Kinase-like folds
DNA-RNA binding domains (helix-turn-helix motifs)
WHat are quaternary structures? What interactions? Two types of proteins?
Multiple protein subunits fold to make big functional complex
Stabilized w/ noncovalent interactions and maybe some disulfide (if cyestinne)
SUbunits are identical → Homomeric
Subunitis are different → heteromeric
What is the hydrophobic efffect? Why and how does it drive protein folding?
ENTROPY-DRIVEN effect
Hydrophobic effect drives protein folding b/c water molecules gain entropy when released after trying to come into contact with the hydrophobic amino acids which are packed into the center of a protein
Increases the entropy of the system
Hydrophobic amino acids pack themselves in middle to minimize exposure to water
Makes process energetically facorable (negative ∆G)
What is the levinthal paradox and how does it lead us to know protein folding is not random
If protein folding was random it would take years for protein to be formed
HOw do we know how proteins know what to fold to? What experiment was connected?
Ribonuclease A (protein) exposed to urea (disrupts H-bonds) and B-mercaptoethanol (disrupts disulfide bonds) causing protein to denature → reagents removed → protein folds back into Ribonuclease A
Protein folding encoded genetically into primary sequence
What does it mean the protein folding is sequence driven? What does it mean?
Means that proteins can be denatured and renatured spontaneously in sollution (outside of the cell)
Shows that protein folding is ingrained into primary genetic sequence
What is cumaltive Selection? How does it allow for correct stable folding?
As things rotate, some atoms come closer together. You eventually get stabilization when positive and negative things come together and bind/
Driven by achieveing lowest possible energy state
avorable local interactions are retained, progressively narrowing the conformational search space.
Do you need to consider the ype of amino acid even if they are of the group for specific interactions?
YES-
Ex: Alanine and leucine. If leucine relaced with alanine in the core of a protein it becomes less stable beacuse leucine is larger and will form more hydrophobic interactions
Clincial Example: What happens if PrPc (a a-helix) comes into contact with prP?
The a-helix PrPc transforms into PrPsc (a B-sheet) and the hydrophobic affects causes the sheets to push together, aggregate, and distrupt cell fun tion
How do you determine Charge of a peptide sequence?
YOU MUST Ignore the a-Amino’s and a-COOH’s because they are forming peptide (amide) bonds between each other → ONLY R groups are considered
REMEMBER TO TAKE INTO ACCOUNT THE N AND C TERMINI