Module 6
1/144
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
145 Terms
What is signal transduction?
The process by which extracellular messages (signals) from outside the cell communicate a response within a target cell or tissue.
What are the different classes of extracellular messengers/signals?
The four main classes of extracellular messengers are:
Hormones - act at a distance (e.g., insulin)
Neurotransmitters - secretion close to target cells (e.g., dopamine)
Pheromones (signals between organisms)
Growth factors - act at various distances (regulate cell growth and division)
How do hormones differ from neurotransmitters?
Hormones travel long distances through the bloodstream to target tissues.
Neurotransmitters act locally and exert their effect quickly.
What is the function of pheromones?
Secreted by one organism and affect another organism, often influencing behavior or physiological responses.
What are the three main types of hormones in vertebrates?
Peptides (e.g., insulin, glucagon)
Steroids (e.g., vitamin D, estrogen)
Amino acid derivatives (e.g., epinephrine, thyroxine)
What are hormones?
Small molecules secreted by endocrine glands which travel through the bloodstream and bind to specific receptors on or in target cells.
Ex: Epinephrine, glucagon, insulin
How does insulin function in signal transduction?
The pancreas releases insulin in response to high blood sugar levels.
Insulin binds to receptors on target cells (muscle and adipose tissue).
This signals the cells to take up glucose for use or storage.
What is the role of epinephrine in the body?
Secreted by the adrenal gland and promotes ATP (energy) production in muscle during exercise.
How does glucagon regulate blood sugar levels?
Secreted by the pancreas when blood glucose is low. It acts on the liver to:
Promote glycogen breakdown
Stimulate gluconeogenesis (glucose production)
What are the key components of signal transduction?
Signal (Messenger) – The molecule that carries the message (e.g., insulin).
Receptor – The protein on/in a target cell that binds to the signal.
Transducer – Converts the signal into a cellular response.
Effector – Carries out the response (e.g., glucose uptake).
What is signal amplification?
When a small amount of a signal molecule triggers a larger, significant cellular response.
What are the three steps of cell signaling (signal transduction)?
Reception – The extracellular signal is received by a receptor.
Transduction – The signal is converted from outside to inside the cell.
Response – The cell carries out the appropriate reaction to the signal.
What is an example of signal transduction involving glucagon?
Glucagon is released by the pancreas when blood glucose levels are low.
It travels through blood stream and binds to glucagon receptors on liver cells.
This triggers the release of glucose from glycogen stores to increase blood sugar levels.
What is a ligand in signal transduction?
A signal molecule (such as a hormone) that binds to a receptor to initiate a response.
What is the role of a G protein in signal transduction?
Acts as a transducer that relays signals inside the cell.
It is a heterotrimeric protein made of three subunits: alpha, beta, and gamma.
It switches between active/dissociative (GTP-bound) and inactive (GDP-bound) states.
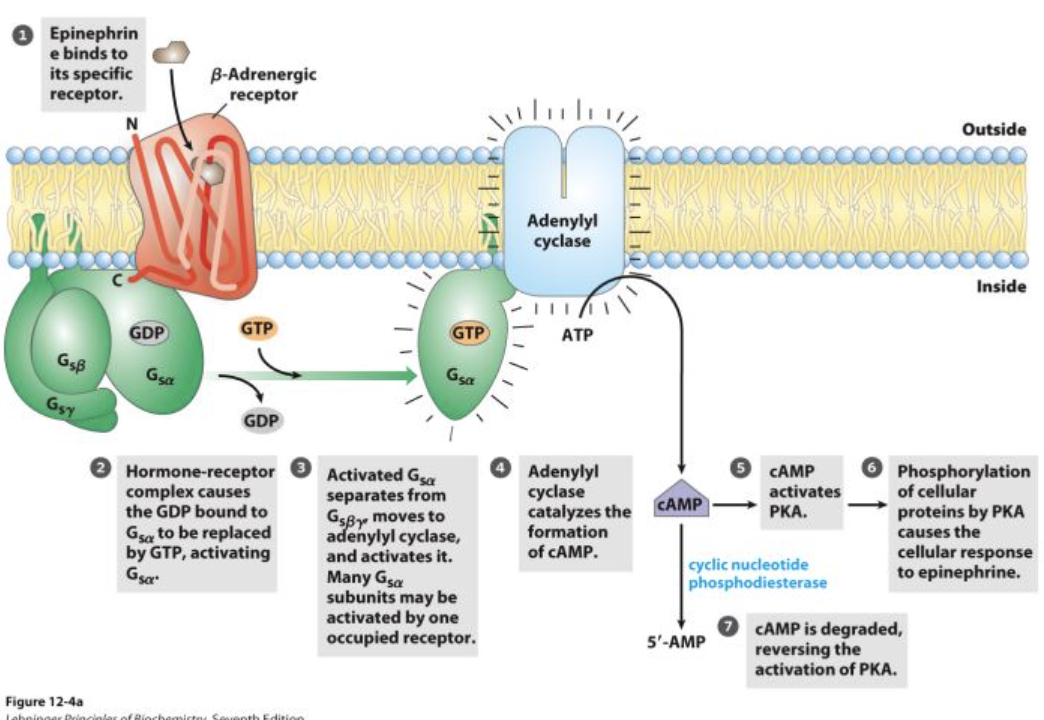
How does epinephrine signal through the beta adrenergic receptor?
Epinephrine (ligand) binds to the beta adrenergic receptor (transmembrane protein).
This causes a conformational change in the receptor.
The receptor interacts with a G protein, exchanging bound GDP for GTP.
The activated G protein then activates adenylate cyclase (effector), leading to the cellular response.
What is the role of GDP and GTP in G protein activation?
When GDP is bound, the G protein is inactive.
When GTP replaces GDP, the G protein is active and can trigger further signaling.
What enzyme does the activated G protein stimulate?
The activated G protein stimulates adenylate cyclase, which helps elicit the cellular response.
What is the role of adenylate cyclase (AC) in signal transduction?
Is an effector enzyme that converts ATP to cAMP, a second messenger, in response to G protein activation.
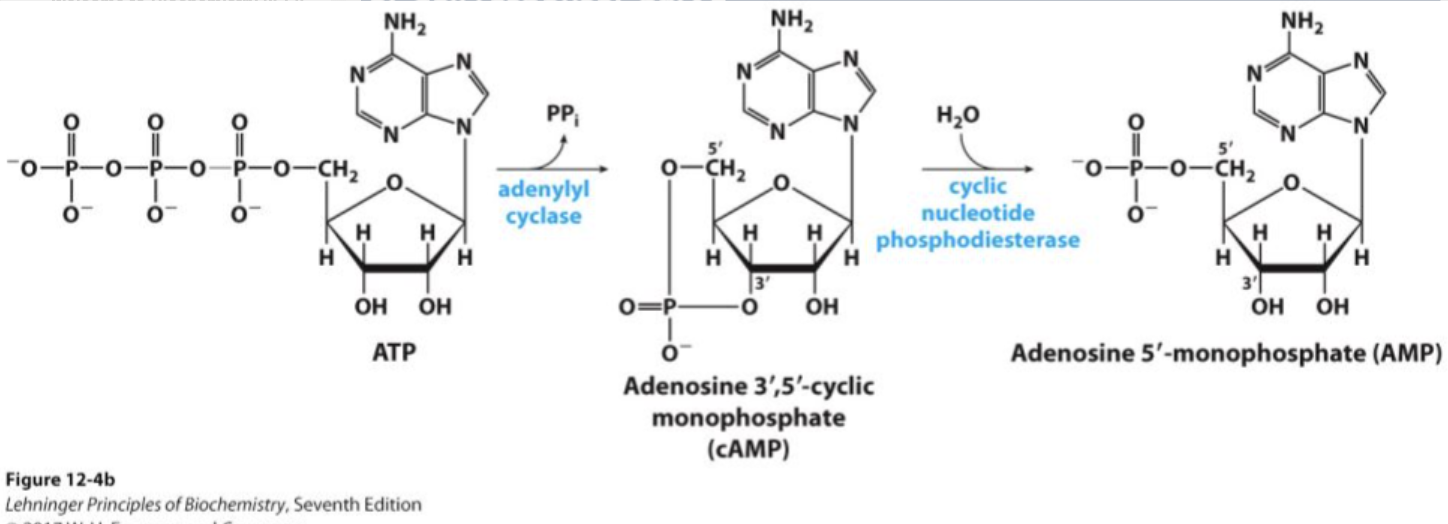
What are the first and second messengers in the epinephrine signaling pathway?
First messenger: Epinephrine (the original signal molecule).
Second messenger: cAMP (transmits the signal inside the cell).
What enzyme does cAMP activate?
Activates protein kinase A (PKA), which starts a phosphorylation cascade.
What happens after protein kinase A is activated?
PKA phosphorylates phosphorylase kinase b, activating it.
Phosphorylase kinase b activates glycogen phosphorylase by phosphorylating it.
Active glycogen phosphorylase a catalyzes glycogen breakdown, releasing glucose for ATP synthesis.
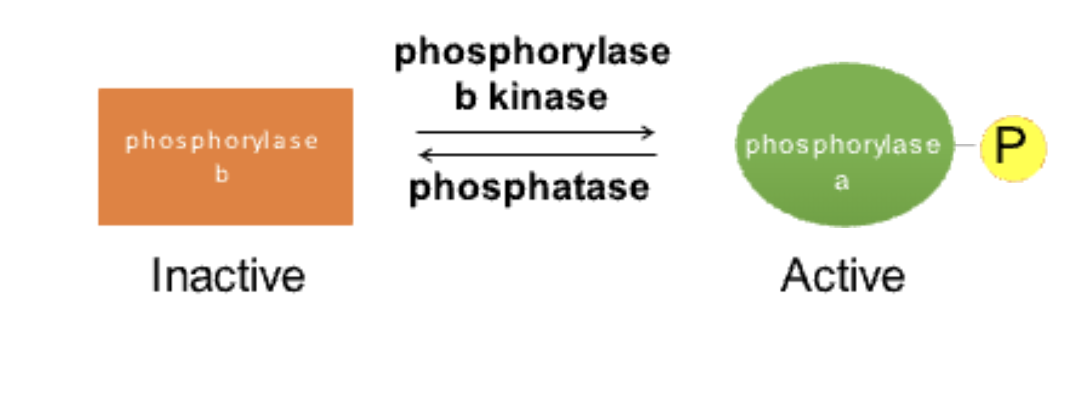
What is the difference between glycogen phosphorylase a and b?
Glycogen phosphorylase a: Active (phosphorylated form).
Glycogen phosphorylase b: Inactive (dephosphorylated form).
Why is the breakdown of glycogen important in epinephrine signaling?
Glycogen breakdown provides glucose-1-phosphate, which is used for ATP production to support muscle contraction during exercise.
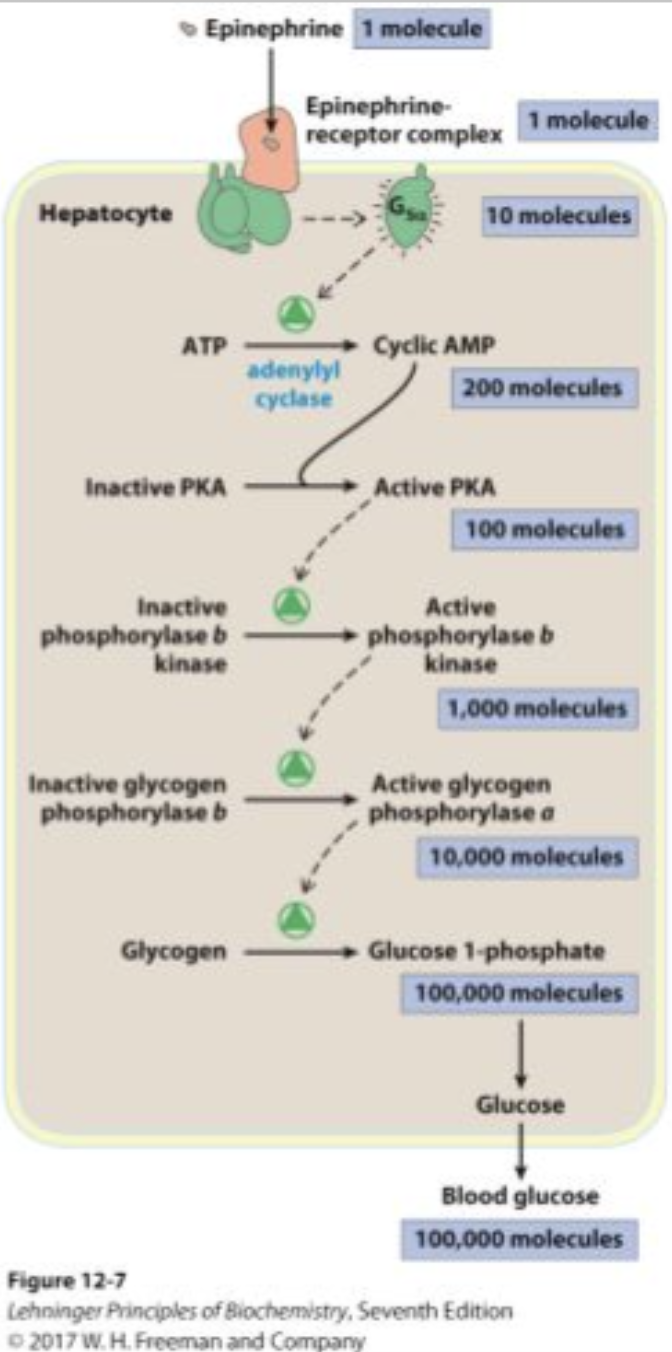
How does signal amplification occur in epinephrine signaling?
One epinephrine molecule activates one receptor.
One receptor activates many G proteins.
Each G protein activates adenylate cyclase, producing many cAMP molecules.
Each cAMP activates multiple PKA enzymes, leading to a cascade that amplifies the signal.
What type of receptor is the beta adrenergic receptor?
The beta adrenergic receptor is a G protein-coupled receptor (GPCR).
What is another major type of receptor involved in signal transduction?
Receptor tyrosine kinases (RTKs), such as the insulin receptor, are another major class of receptors.
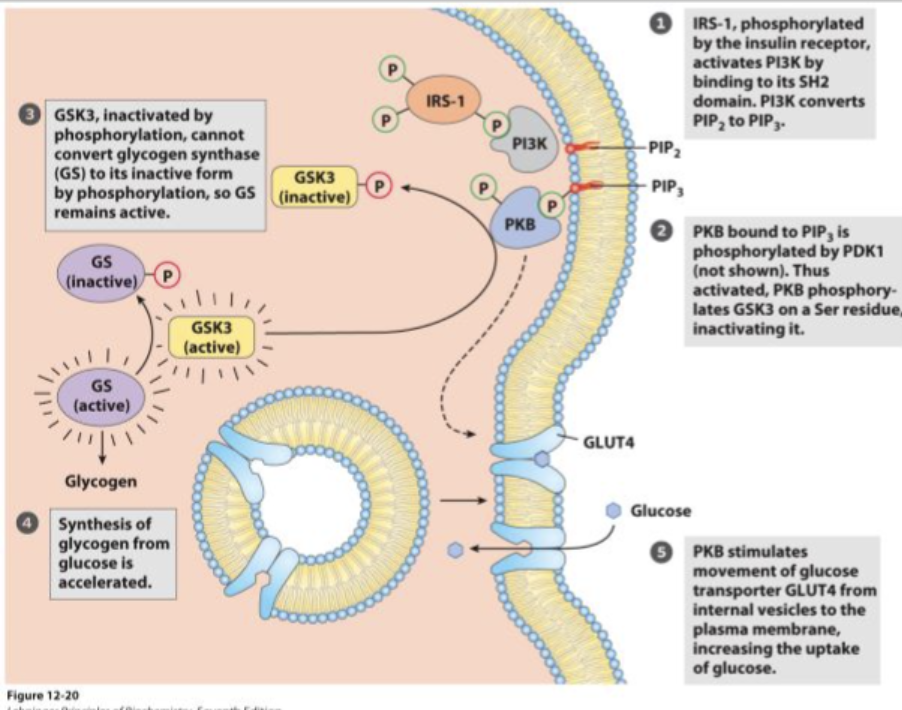
How does the insulin receptor signal transduction pathway work?
Insulin binds to its receptor (RTK), activating tyrosine kinase activity.
This leads to a cascade that synthesizes PIP3 (a second messenger).
The final response is the relocation of GLUT4 transporters to the membrane, allowing glucose uptake from the bloodstream.
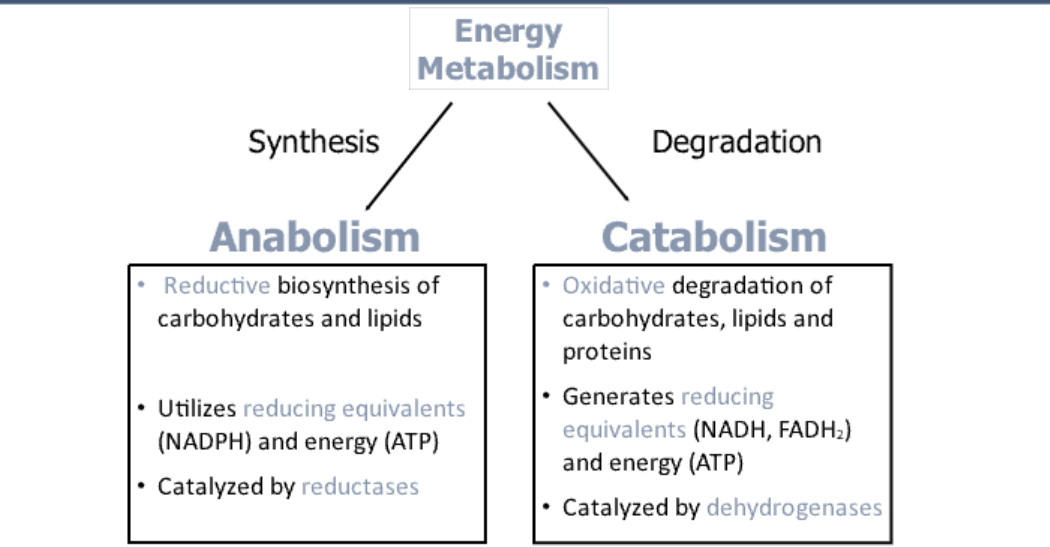
What is energy metabolism?
The pathways involved in the generation or storage of metabolic energy.
Why do we need energy metabolism?
Provides the fuel our body needs to move, function, and survive, using energy from food or stored reserves.
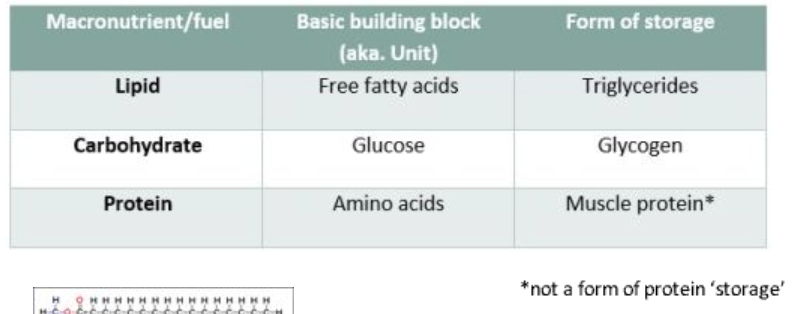
What are the three macronutrients used for energy?
Carbohydrates (sugars)
Lipids (fats)
Proteins
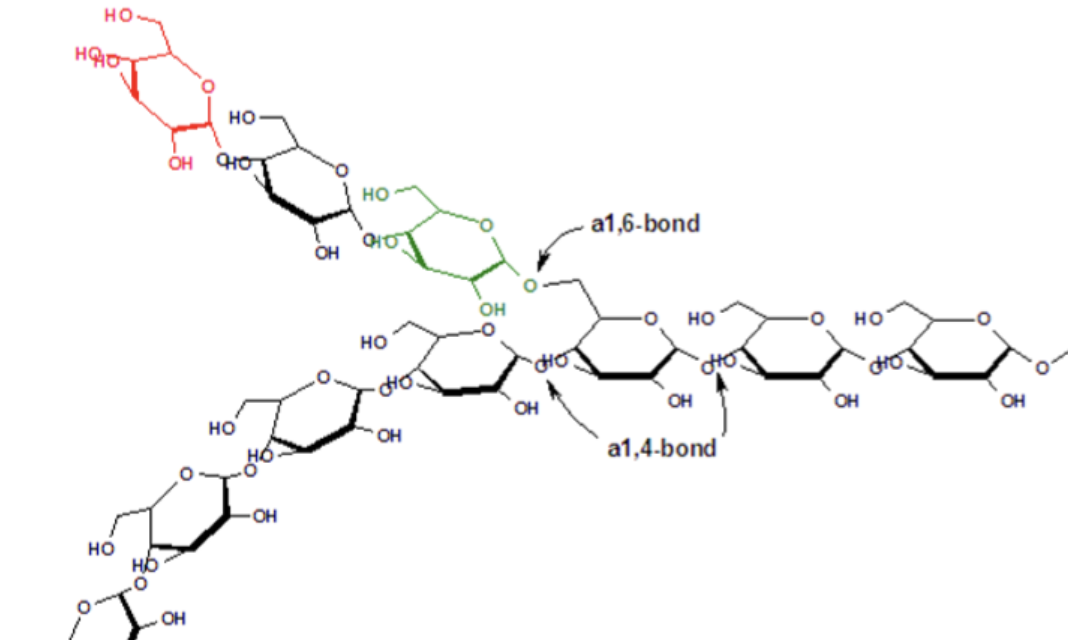
How are carbohydrates stored in the body?
Stored as glycogen, a large molecule made of glucose units.
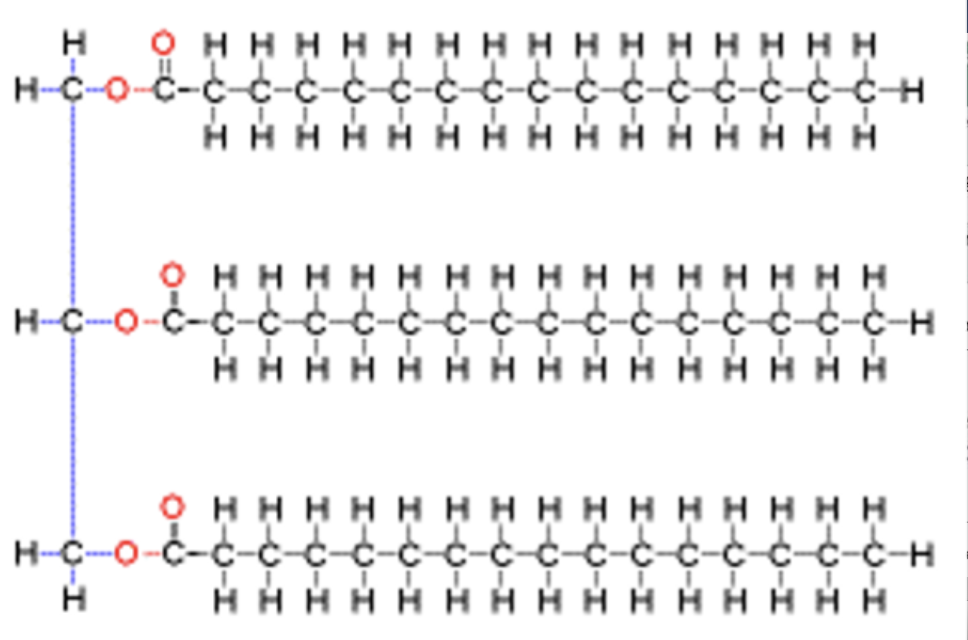
How are lipids stored in the body?
Stored as triglycerides, which consist of three fatty acids attached to glycerol.
How are proteins stored in the body?
The body does not store protein in a way similar to carbohydrates or lipids. However, during starvation, the body can break down muscle protein for energy.
What is the purpose of energy metabolism?
Breaks down macronutrients to generate energy, primarily in the form of ATP.
What is ATP and why is it important?
ATP (Adenosine Triphosphate) is the energy currency of the cell. It is broken down into ADP and Pi (inorganic phosphate) to release energy for cellular functions.
Why is breakfast important for energy metabolism?
Provides glucose, which fuels the brain and muscles, helping with physical activity and learning.
What is the brain’s preferred fuel source?
The brain prefers glucose as its primary fuel source.
What does muscle use for energy?
During high-intensity exercise, muscles use glucose.
During rest or low-intensity exercise, muscles use lipids (fats).
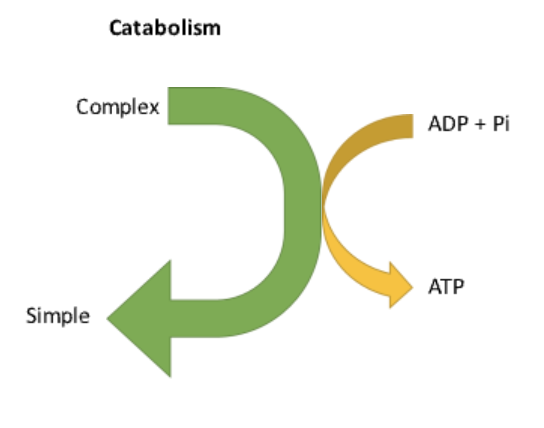
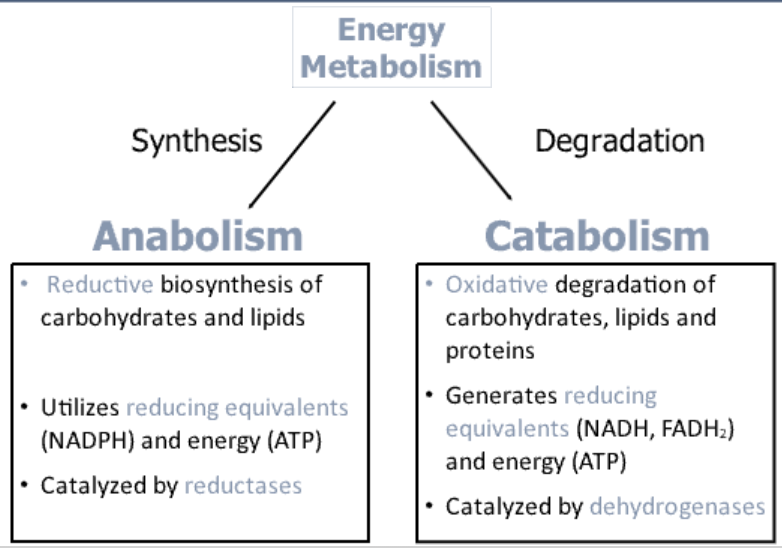
What is catabolism?
The breakdown of complex macronutrients into smaller molecules, releasing ATP.
Ex: The breakdown of glycogen into glucose for energy.
What are examples of catabolic pathways?
Glycolysis: Glucose → Pyruvate
Citric Acid Cycle: Acetyl-CoA → Carbon dioxide + ATP
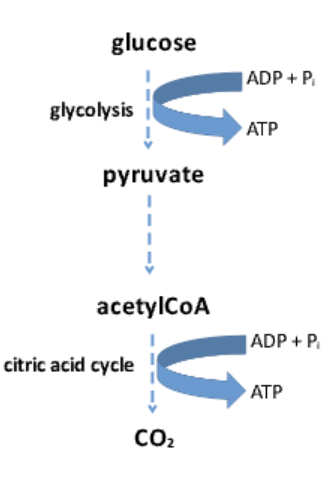
How does glycolysis generate energy?
Breaks down glucose into pyruvate, generating ATP in the process.
What happens to pyruvate after glycolysis?
Pyruvate can be further broken down into Acetyl-CoA, which enters the Citric Acid Cycle to produce more ATP.
What is anabolism?
The process of building complex molecules from simpler ones, requiring energy input.
Ex: The synthesis of proteins from amino acids.
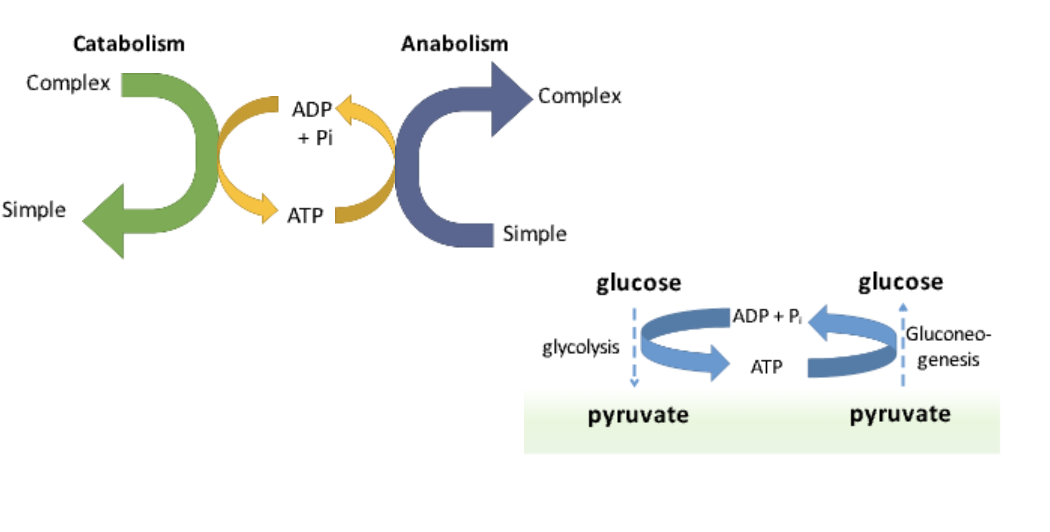
What is an example of an anabolic pathway?
Gluconeogenesis, which converts pyruvate into glucose.
What are the two main types of metabolism?
Catabolism: Breakdown of macromolecules → Energy release (ATP)
Anabolism: Synthesis of macromolecules → Requires energy (ATP use)
What is a metabolic pathway?
A series of sequential reactions in a cell, each catalyzed by a specific enzyme.
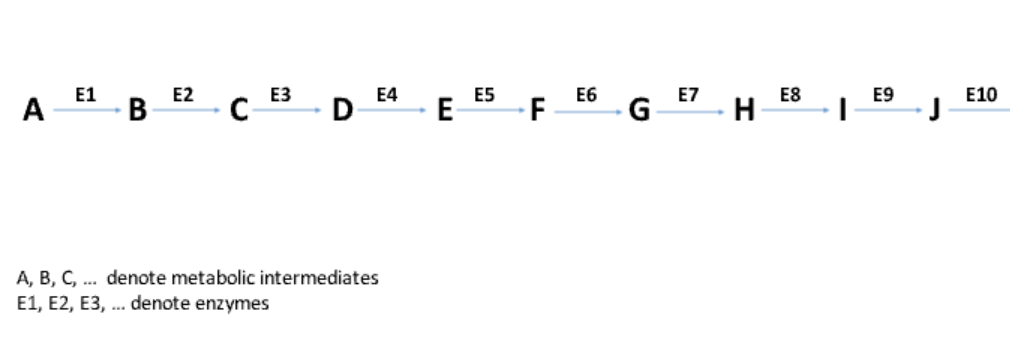
What are metabolic intermediates?
The molecules in a pathway that are transformed into different compounds through enzyme-catalyzed reactions.
Energy Metabolism Chart
What is an example of a metabolic pathway?
Glycolysis, which consists of 10 reactions that break down glucose to generate energy.
How does catabolism involve oxidation?
An oxidative process where electrons are removed from macronutrients and transferred to coenzymes like NAD+, NADP+, and FAD.
What type of enzymes catalyze oxidation reactions in catabolism?
Dehydrogenases catalyze oxidation reactions by removing electrons and donating them to coenzymes.
What happens to the electrons from reduced coenzymes?
Electrons from NADH and FADH2 are donated to the electron transport chain to convert ADP into ATP.
How does anabolism involve reduction?
Is a reductive process, where electrons are donated by reduced coenzymes (NADH, NADPH) to synthesize complex molecules.
What type of enzymes catalyze reduction reactions in anabolism?
Reductases catalyze reduction reactions, helping in biosynthesis (e.g., fatty acid synthesis).
Textbook
…
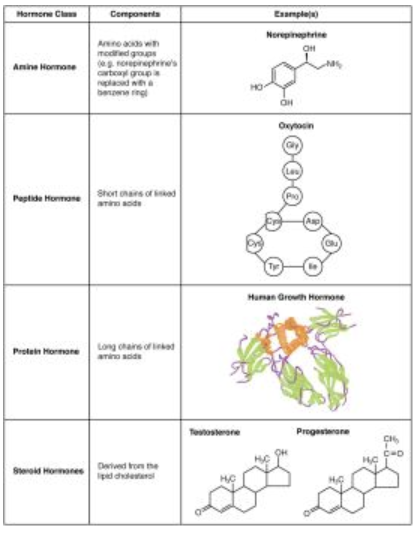
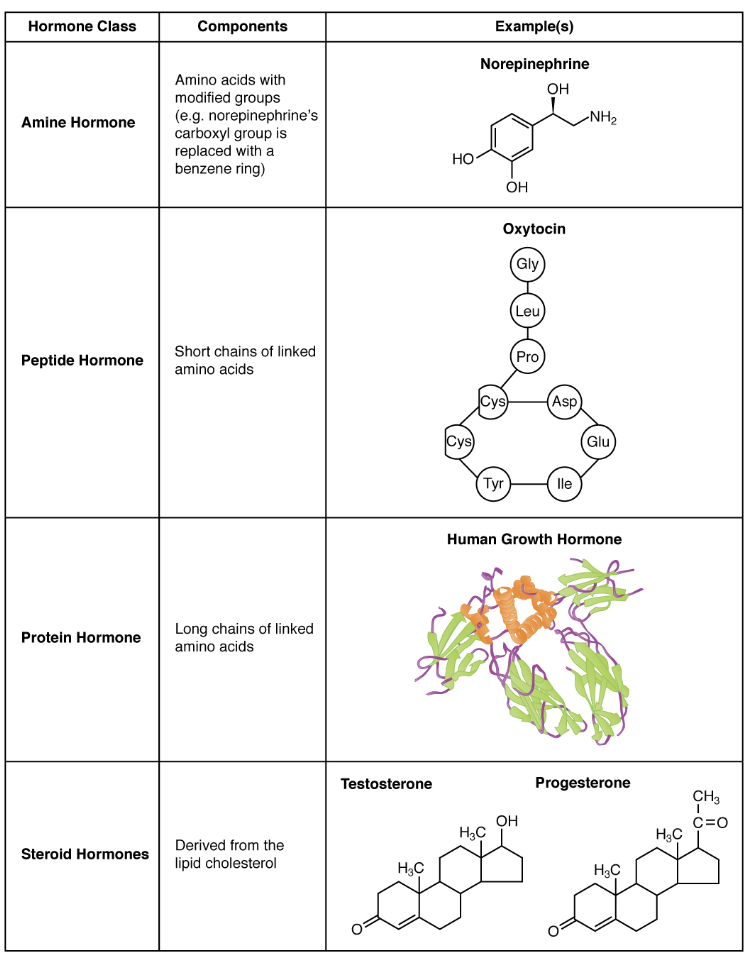
What are the three major classes of hormones based on chemical structure?
Amine Hormones – Derived from amino acids with modified groups (e.g., epinephrine, melatonin).
Peptide and Protein Hormones – Made of amino acid chains - short vs. long (e.g., insulin, growth hormone).
Steroid Hormones – Derived from the lipid cholesterol (e.g., testosterone, cortisol).
What are the major hormones of the anterior pituitary and their functions?
Growth Hormone (GH) – Promotes body tissue growth.
Prolactin (PRL) – Stimulates milk production.
Thyroid-Stimulating Hormone (TSH) – Stimulates thyroid hormone release.
Adrenocorticotropic Hormone (ACTH) – Stimulates adrenal cortex hormones.
Follicle-Stimulating Hormone (FSH) – Stimulates gamete production.
Luteinizing Hormone (LH) – Stimulates androgen production in gonads.
What are the major hormones of the posterior pituitary and their functions?
Antidiuretic Hormone (ADH) – Stimulates water reabsorption in kidneys.
Oxytocin – Stimulates uterine contractions and milk ejection.
How do lipid-soluble and water-soluble hormones differ in their action?
Lipid-Soluble Hormones (e.g., steroids) cross the cell membrane and bind to intracellular receptors, directly affecting gene transcription.
Water-Soluble Hormones (e.g., peptides) bind to membrane receptors and use second messengers (e.g., cAMP) to trigger responses.
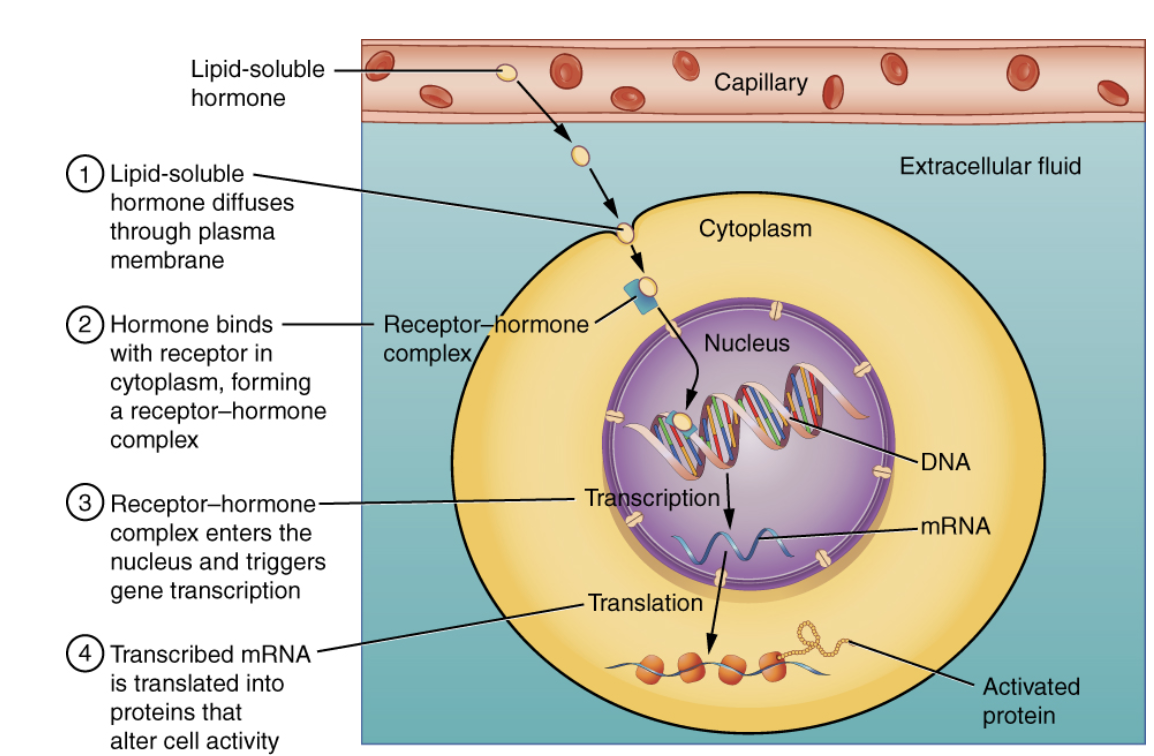
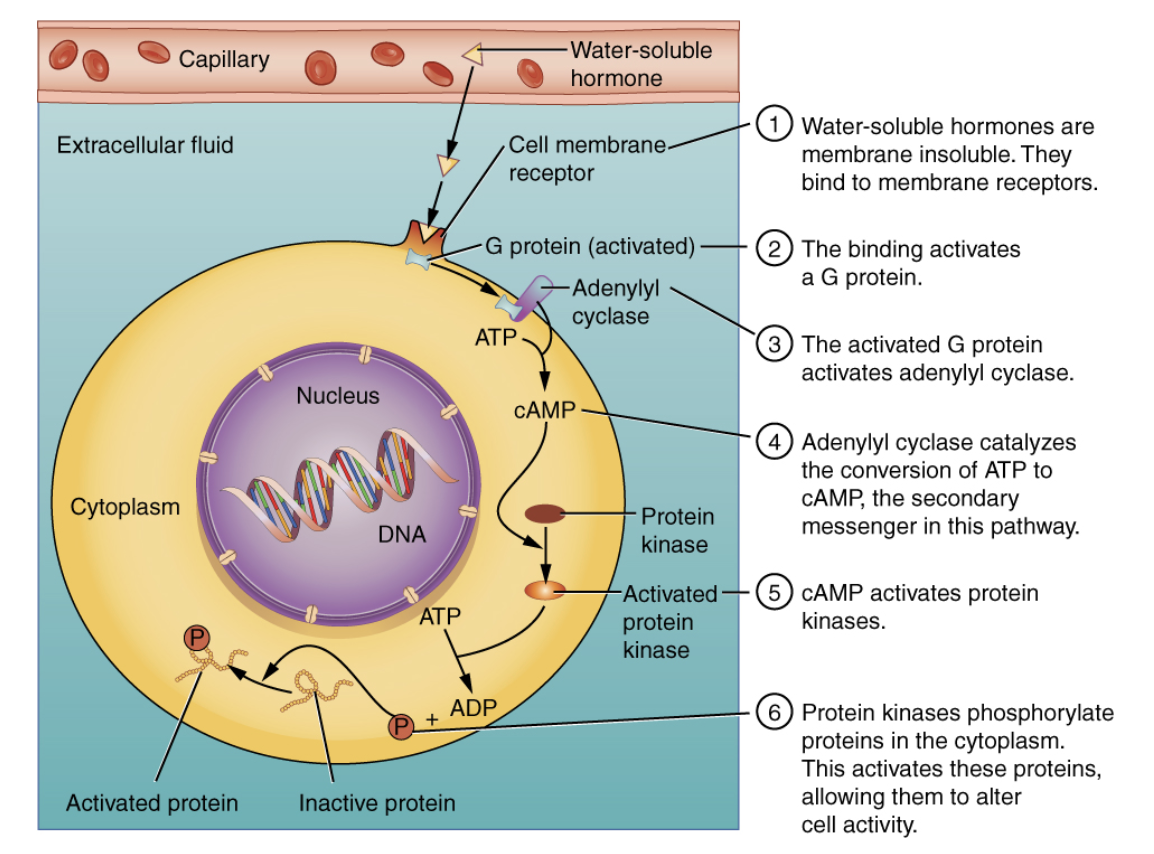
What is the role of intracellular hormone receptors?
Found inside the cell.
Bind lipid-soluble hormones (steroids, thyroid hormones).
Form hormone-receptor complexes that directly influence DNA transcription and protein synthesis.
What is the role of cell membrane hormone receptors?
Found on the cell surface.
Bind water-soluble hormones (peptides, proteins).
Activate second messenger systems (e.g., cAMP, IP3).
How does the cAMP signaling pathway work for water-soluble hormones?
Hormone binds to receptor.
G-protein activates adenylyl cyclase.
Adenylyl cyclase converts ATP to cAMP (second messenger).
cAMP activates protein kinases, triggering a cellular response.
What is an alternative second messenger system involving calcium (IP3)?
Some hormones use IP3 and DAG instead of cAMP.
IP3 causes calcium release inside the cell, activating proteins like calmodulin.
DAG activates protein kinases, leading to cellular changes.
What are the three major hormone interactions?
Permissive Effect – One hormone enables another (e.g., thyroid hormones aid reproductive hormones).
Synergistic Effect – Two hormones amplify the response (e.g., FSH + estrogen for egg maturation).
Antagonistic Effect – Two hormones have opposite actions (e.g., insulin lowers blood sugar, glucagon raises it).
What are upregulation and downregulation of hormone receptors?
Upregulation – Cells increase receptor numbers when hormone levels are low.
Downregulation – Cells decrease receptor numbers when hormone levels are high.
What are the two types of feedback loops in hormone regulation?
Negative Feedback – Most common; inhibits further hormone release when levels are sufficient (e.g., glucocorticoid regulation by the hypothalamus & pituitary).
Positive Feedback – Enhances hormone release in response to initial stimulus (e.g., oxytocin during childbirth).
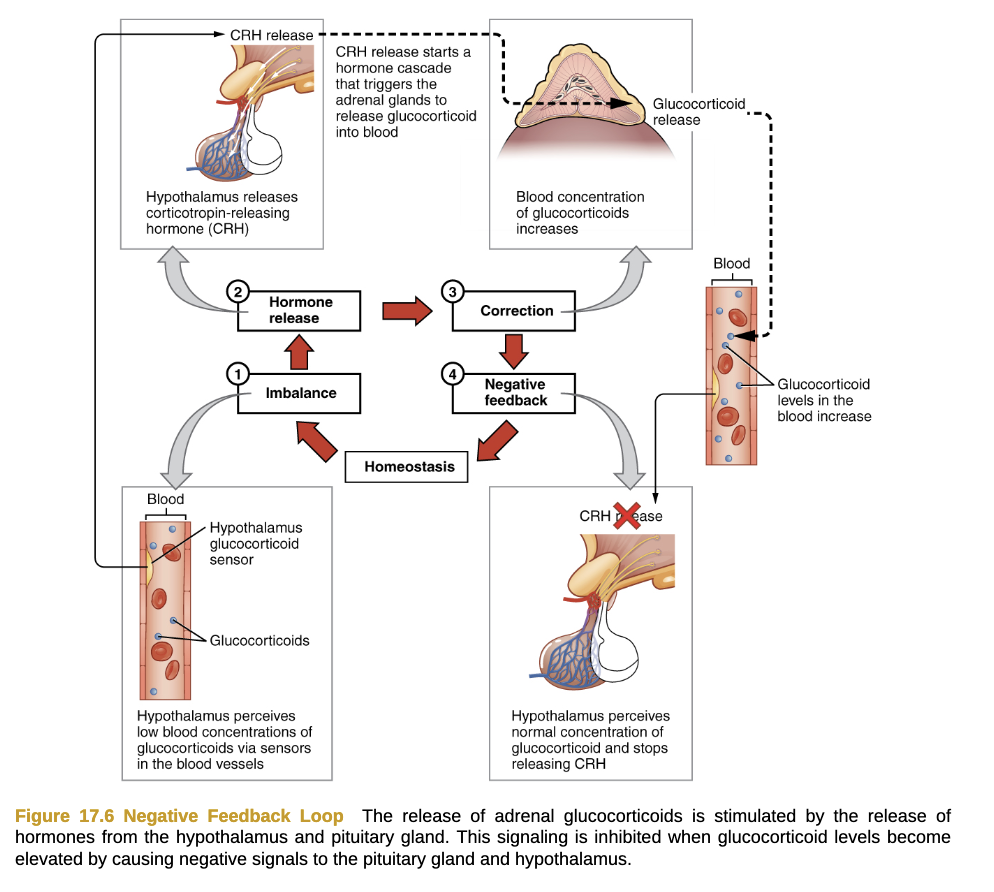
What are the three types of endocrine stimuli?
Humoral Stimuli – Changes in blood levels of ions/nutrients (e.g., low blood Ca²⁺ triggers PTH release).
Hormonal Stimuli – Hormones triggering other hormones (e.g., TSH stimulates thyroid hormones).
Neural Stimuli – Nerve signals triggering hormone release (e.g., stress triggers adrenal medulla to release epinephrine).
GCPR - What are G-protein-coupled receptors (GPCRs), and why are they significant in biology and medicine?
GPCRs are the largest and most diverse group of membrane receptors in eukaryotes. They detect external signals like light, peptides, and hormones, triggering cellular responses. They play a crucial role in many physiological functions, and up to half of all marketed drugs target GPCRs.
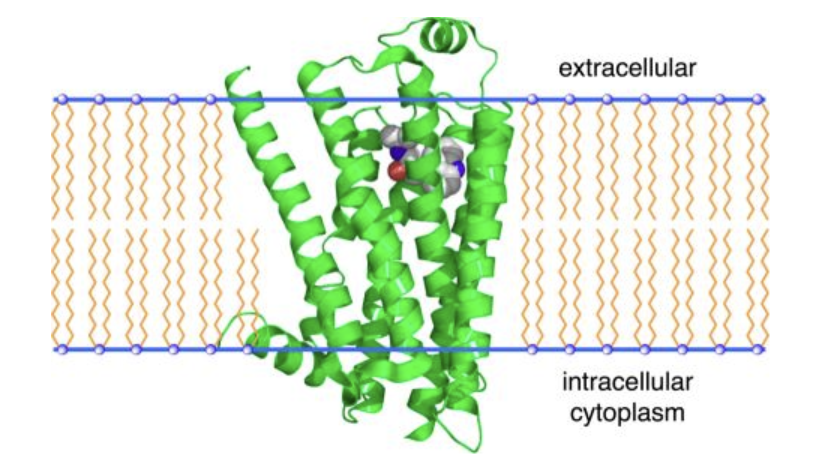
GCPR - What is the common structural feature of GPCRs?
GPCRs consist of a single polypeptide with seven transmembrane segments spanning the plasma membrane. Their extracellular loops form binding pockets for signaling molecules, while intracellular regions interact with G proteins to transmit signals.
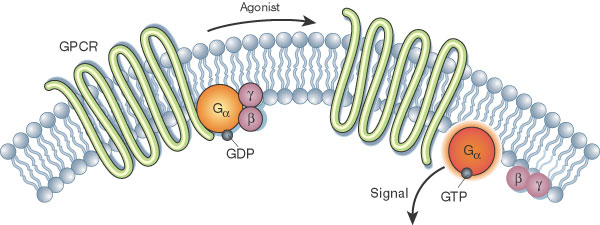
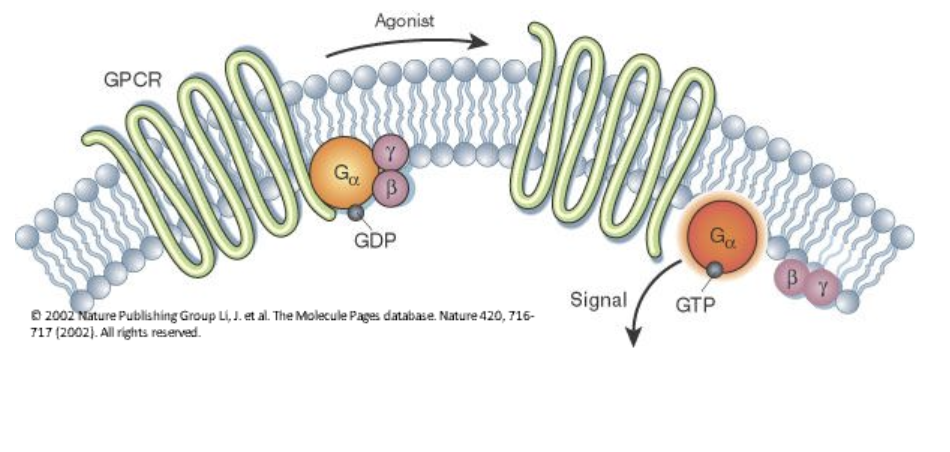
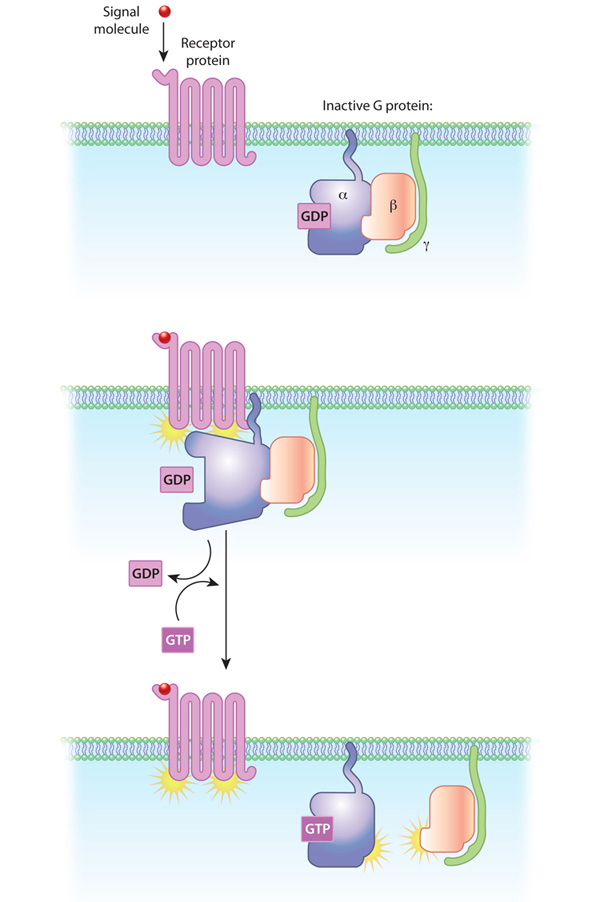
GCPR - Describe how GPCRs activate G proteins upon ligand binding.
When a ligand binds to a GPCR, the receptor undergoes a conformational change, activating a nearby G protein. This causes GDP (inactive) on the G protein’s alpha subunit to be replaced by GTP (active), leading to the dissociation of the alpha subunit and beta-gamma dimer, which then interact with other cellular targets to propagate the signal.
GCPR - What are the major second messengers triggered by GPCR signaling?
GPCR activation can lead to the production of second messengers like:
cAMP (via adenylyl cyclase) – involved in hormone responses and nerve signaling.
DAG & IP3 (via phospholipase C) – critical for processes like blood clotting and intracellular calcium signaling.
GCPR - How do G proteins function as molecular switches in GPCR signaling?
G proteins act as switches that turn on when bound to GTP and off when GTP is hydrolyzed back to GDP. The active GTP-bound alpha subunit and beta-gamma dimer trigger intracellular signaling cascades. When GTP is hydrolyzed, the subunits reassociate, resetting the system.
What are the key characteristics of signal transduction?
Signal transduction is specific and highly sensitive. Specificity is due to molecular complementarity between signals and receptors. Sensitivity is due to high receptor affinity, amplification through cascades, and cooperative ligand binding.
How do multicellular organisms achieve specificity in signal transduction?
Different cell types have specific receptors and intracellular targets for signals. For example, thyrotropin-releasing hormone affects anterior pituitary cells but not hepatocytes, which lack the receptor.
What mechanisms contribute to the high sensitivity of signal transduction?
High receptor affinity (low Kd, often ≤10⁻⁷ M) allows detection of low ligand concentrations.
Cooperativity in ligand binding increases response to small concentration changes.
Amplification through enzyme cascades rapidly increases signal strength.
What is the role of modularity in signal transduction?
Modular signaling proteins can bind multiple partners, allowing different pathways to mix and match components. Scaffold proteins bring enzymes together for efficient signaling.
How does desensitization affect receptor sensitivity?
Continuous exposure to a signal reduces receptor responsiveness. When the stimulus drops below a threshold, sensitivity is restored. (Example: adjusting to light changes when moving between bright and dark environments.)
What is signal integration?
Signal integration allows a cell to receive multiple signals and generate a coordinated response. Different pathways interact through cross-talk to maintain cellular homeostasis.
What are the four major types of receptors in signal transduction?
G protein–coupled receptors (GPCRs): Indirectly activate enzymes via G proteins (e.g., β-adrenergic receptor).
Receptor enzymes: Have intracellular enzyme activity (e.g., insulin receptor with tyrosine kinase activity).
Gated ion channels: Open/close in response to ligands or voltage changes.
Nuclear receptors: Bind steroid hormones to regulate gene expression.
What is response localization in signaling?
Signals are confined to specific subcellular locations (e.g., lipid rafts in membranes), allowing local control without affecting the entire cell.
What are some common biological signals?
Cells respond to various signals, including:
Hormones (e.g., insulin, epinephrine)
Neurotransmitters
Growth factors
Odorants, tastants
Light, mechanical touch
What are key conserved elements in signaling systems?
GPCRs (7TM receptors)
G proteins (bind GTP/GDP)
Protein kinases (phosphorylate target proteins)
Membrane enzymes (produce cyclic nucleotides like cAMP)
Ca²⁺-binding proteins
What are the general steps in signal transduction?
Ligand binds receptor.
Activated receptor triggers intracellular signaling.
Signal amplification occurs.
Cellular response happens (e.g., gene expression, metabolism change).
Signal is terminated to prevent overstimulation.
What are the three essential components of G Protein–Coupled Receptors (GPCRs) signal transduction?
Plasma membrane receptor – Has seven transmembrane helices.
G protein – Cycles between active (GTP-bound) and inactive (GDP-bound) forms.
Effector enzyme/ion channel – Regulated by the G protein to alter cellular responses.
How do GPCRs transmit a signal inside the cell?
A ligand (first messenger) binds to the GPCR.
The GPCR undergoes an allosteric transition and activates a G protein by exchanging GDP for GTP.
The activated G protein dissociates and binds to an effector enzyme (e.g., adenylyl cyclase), altering its activity.
The effector enzyme changes the concentration of a second messenger (e.g., cAMP or Ca²⁺).
The second messenger activates downstream targets, like protein kinases, to trigger cellular responses.
What are some medical conditions linked to GPCRs?
GPCRs are involved in allergies, depression, blindness, diabetes, cardiovascular diseases, and cancer (mutations found in 20% of all cancers).
More than one-third of pharmaceuticals target GPCRs.
Example: Beta-blockers target the β-adrenergic receptor to treat hypertension, cardiac arrhythmia, glaucoma, anxiety, and migraines.
What is the function of the β-adrenergic receptor in epinephrine signaling?
Epinephrine binds to the β-adrenergic receptor in muscle, liver, and adipose tissue.
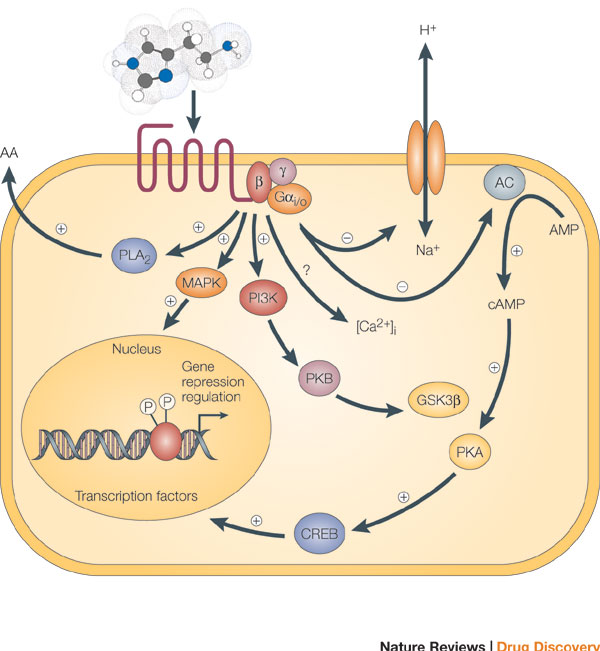
This triggers a G protein cascade that activates adenylyl cyclase, increasing cAMP levels.
cAMP activates Protein Kinase A (PKA), which phosphorylates enzymes involved in glycogen breakdown and energy mobilization ("fight or flight" response).
What are agonists and antagonists in GPCR signaling?
Agonists: Molecules that bind and activate the receptor, mimicking the natural ligand.
Antagonists: Molecules that bind but do not activate the receptor, blocking the effects of the natural ligand.
Example:
Isoproterenol (synthetic agonist) has a higher affinity than epinephrine.
Propranolol (synthetic antagonist) blocks the β-adrenergic receptor.
How does adenylyl cyclase function in GPCR signaling?
Adenylyl cyclase is an enzyme in the plasma membrane that synthesizes cAMP from ATP.
It is activated by Gsα (stimulatory G protein) when bound to GTP.
The cAMP produced acts as a second messenger, activating Protein Kinase A (PKA).
What happens when G proteins switch between active and inactive states?
Inactive state: GDP is bound to the α subunit.
Activation: GPCR triggers GDP-to-GTP exchange on G protein.
Active state: G protein dissociates and interacts with an effector enzyme (e.g., adenylyl cyclase).
Deactivation: The GTPase activity of the G protein hydrolyzes GTP back to GDP, returning it to the inactive state.
How does cAMP activate Protein Kinase A (PKA)?
PKA exists as an inactive tetramer (R₂C₂ complex: 2 regulatory + 2 catalytic subunits).
cAMP binds to the regulatory (R) subunits, causing a conformational change.
The R subunits release the catalytic (C) subunits, activating PKA.
Active PKA phosphorylates target proteins, regulating glycogen metabolism and other cellular processes.
How does fluorescence resonance energy transfer (FRET) help study GPCR signaling?
FRET measures protein-protein interactions in living cells.
It works by detecting energy transfer between fluorescently tagged proteins.
Example:
If PKA’s regulatory and catalytic subunits are close, FRET signal is high.
When cAMP levels rise, PKA dissociates, and FRET signal decreases, indicating PKA activation.
What is the process of β-adrenergic receptor desensitization?
The β-adrenergic receptor is desensitized by phosphorylation. The β-adrenergic receptor kinase (βARK) phosphorylates Ser residues near the receptor's carboxyl terminus. This creates a binding site for β-arrestin, which blocks the receptor's interaction with the G protein and causes the receptor to be internalized through endocytosis. The receptor is eventually dephosphorylated and returned to the membrane, resensitizing it to epinephrine.
What is the role of β-arrestin in the desensitization of the β-adrenergic receptor?
β-arrestin binds to the phosphorylated β-adrenergic receptor, preventing the receptor from interacting with the G protein. This binding also promotes receptor internalization through endocytosis, removing the receptor from the plasma membrane and making it inactive. The receptor is later dephosphorylated and returned to the membrane to be resensitized.
How do β-adrenergic receptors and arrestins contribute to signaling pathways?
When the β-adrenergic receptor interacts with β-arrestin, it triggers a second signaling pathway, specifically the MAPK cascade, in addition to the G-protein signaling pathway. This dual signaling mechanism can be differentially activated by various agonists, and it has important implications for drug development. For example, opioids that favor the G-protein pathway are more addictive, while drugs that favor the arrestin pathway might offer therapeutic effects.
What is the role of cyclic AMP (cAMP) in cellular signaling?
cAMP acts as a second messenger in various signaling pathways, activating protein kinase A (PKA). For example, epinephrine increases cAMP production, which activates PKA. PKA then phosphorylates target proteins, altering their activity. cAMP is also involved in the regulation of gene expression and the synthesis of various hormones, such as cortisol.
How does cyclic AMP (cAMP) influence protein phosphorylation?
cAMP activates protein kinase A (PKA), which then phosphorylates proteins on serine or threonine residues. This phosphorylation alters the activity of these proteins, enabling cellular responses to external signals, such as hormones like glucagon and epinephrine.
How do AKAPs (A kinase anchoring proteins) contribute to the signaling process?
AKAPs are adaptor proteins that bind PKA and anchor it to specific regions within the cell, such as microtubules or the nucleus. This localization ensures that cAMP and PKA have a localized and brief effect, enhancing the specificity of the signal and limiting its duration. Different AKAPs are present in different cell types, leading to tissue-specific responses.
What is the role of phosphodiesterases in cAMP signaling?
Phosphodiesterases break down cAMP into its inactive form, 5′-AMP. This terminates the cAMP-mediated signaling, inactivating PKA and stopping the cellular response to the original signal, such as epinephrine.