3.1.7 Oxidation, reduction and redox equations
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Oxidation
The loss of electrons
Oxidation agents
Electrons acceptors and hence they get reduced
Reduction
The gain of electrons
Reducing agents
Electron donors and hence they get oxidised
Oxidation states
The total number of electrons an element has donated or accepted
Rules for oxidation states - uncombined elements
Uncombined elements have an oxidation state of 0
Elements only bonded to identical atoms have an oxidation state of 0

Rules for oxidation states - monoatomic ions
The oxidation state of a simple monoatomic ion is the same as its charge
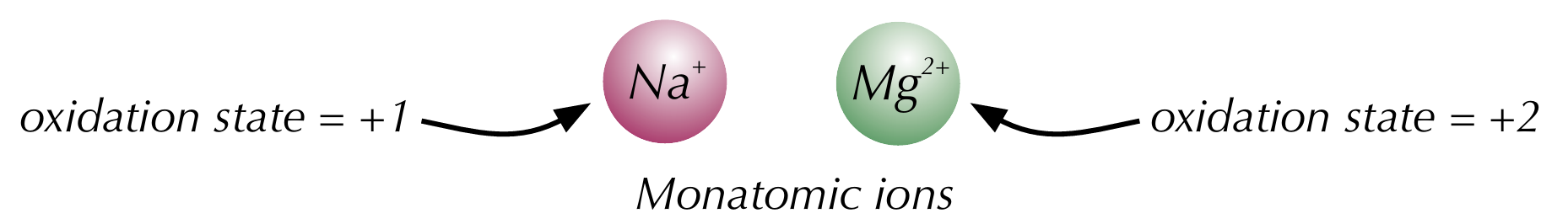
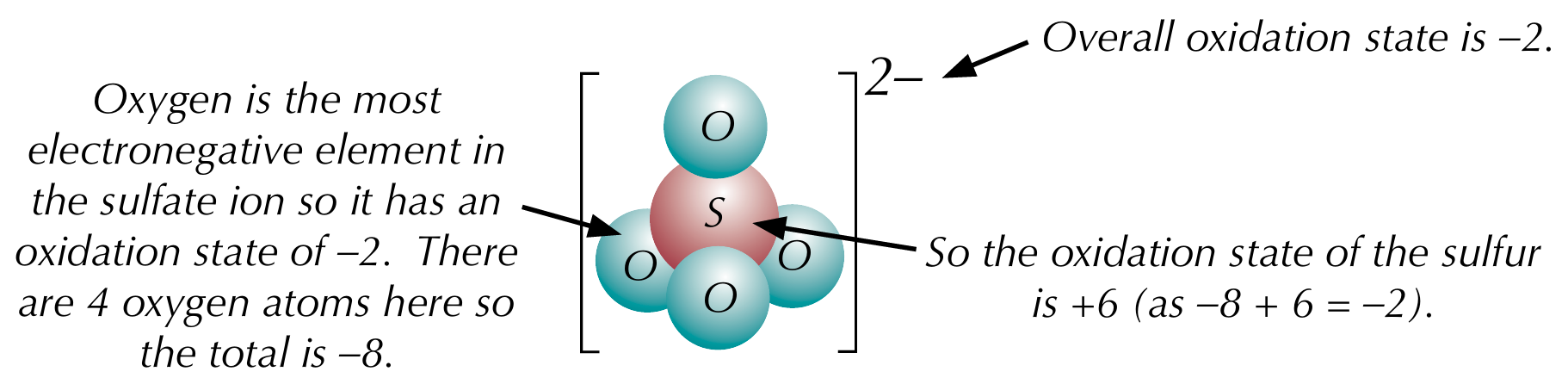
Rules for oxidation states - compounds
The sum of oxidation states is equal to the overall oxidation state which is equal to the charge of the ion
The most electronegative element has a negative oxidation state (equal to its ionic charge), and other elements have more positive oxidation states
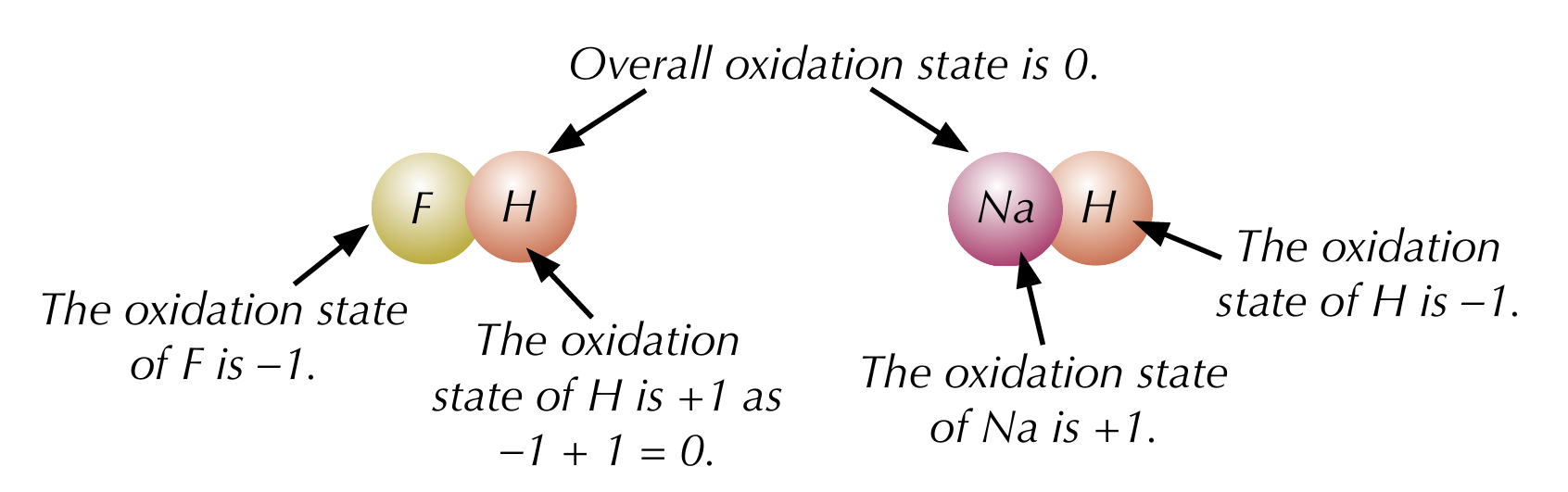
Rules for oxidation states - oxygen
Combined oxygen has an oxidation state of -2, except in peroxides, where it is -1
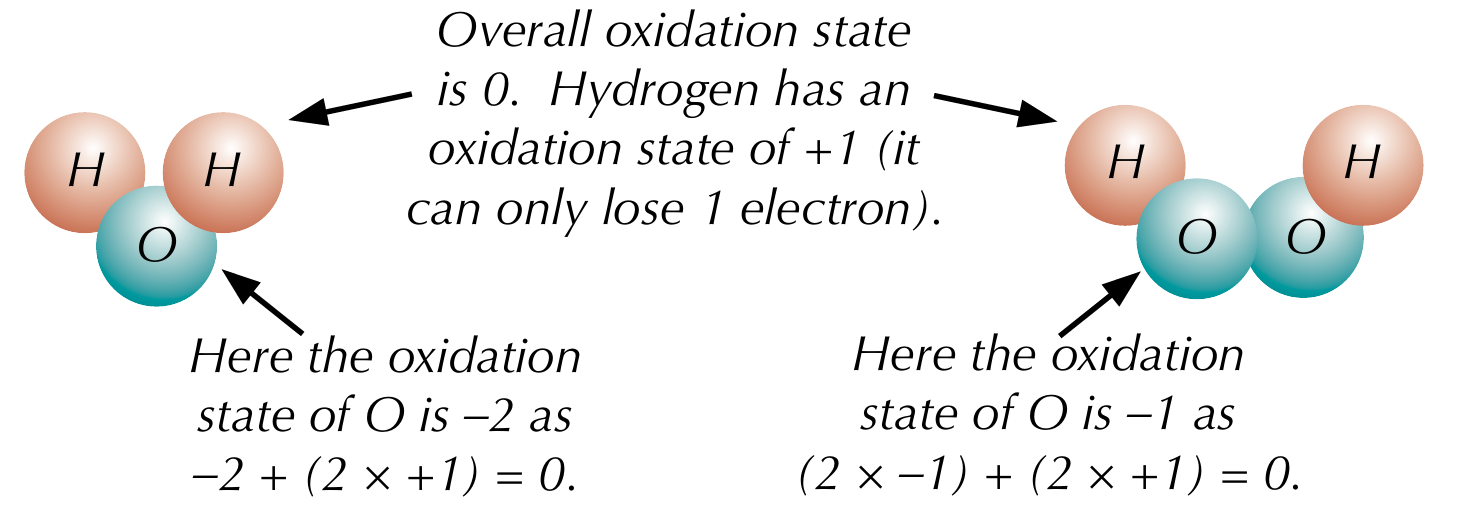
Rules for oxidation states - hydrogen
Combined hydrogen has an oxidation state of +1, except in metal hydrides, where it is 0
Redox reaction
A reaction where reduction and oxidation happen simultaneously
Half-equation
An ionic equation that shows oxidation or reduction — one half of a full redox equation
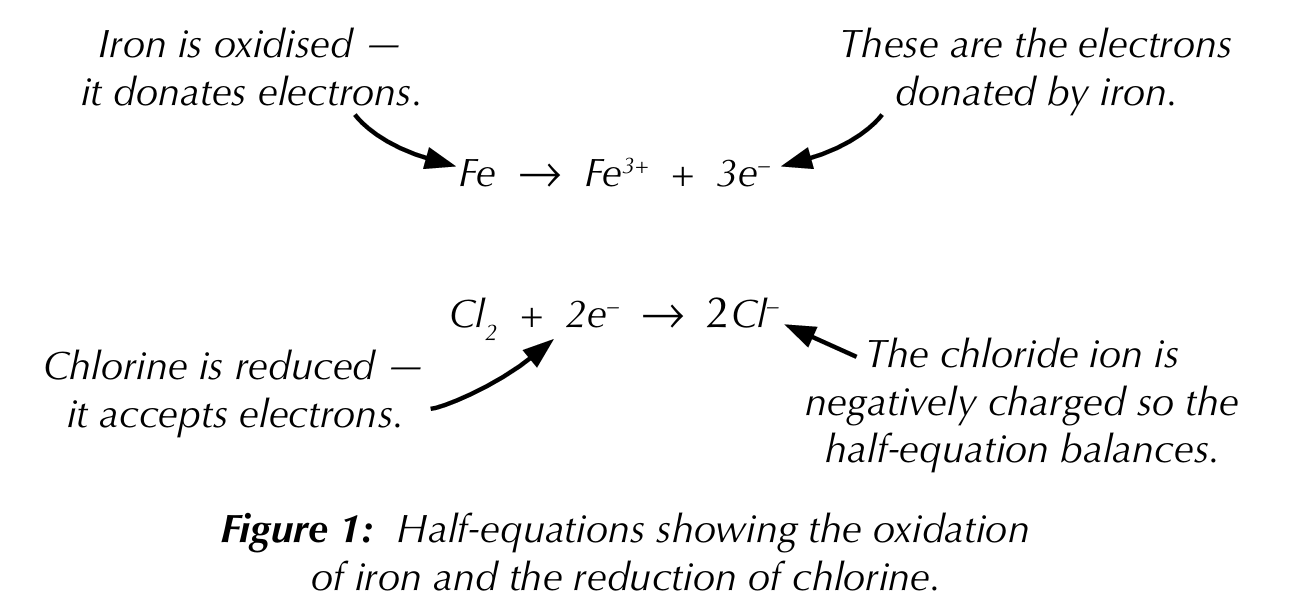
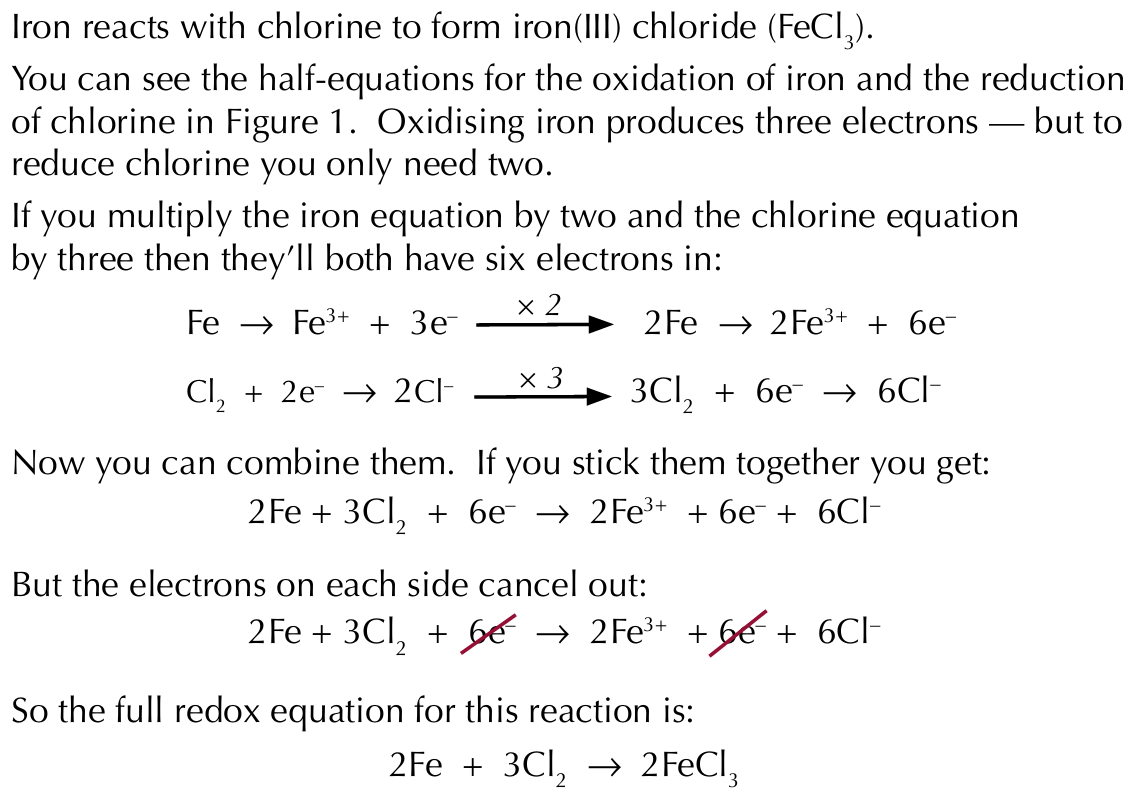
Full equations for redox reactions