PANCE Blueprint Renal System - All Smarty PANCE Renal System Flashcard Lesson Sets Combined (Smarty PANCE)
1/253
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
254 Terms
What are the characteristics of glomerular disease?
Proteinuria, hematuria (microscopic or gross), renal insufficiency, and HTN. Depending upon the cause, systemic symptoms may also be present.
A 10 y/o is brought to the clinic with her mother for dark urine. The mother mentions that the child was complaining of sore throat and cough/cold symptoms two ago. The urine shows gross hematuria without nitrites or leukocytes.
Postinfectious (Poststreptococcal) glomerulonephritis
What is the typical clinical presentation of postinfectious GN?
Presentation varies from asymptomatic with only microscopic findings on urinalysis to that of a nephritic syndrome: gross hematuria, HTN, edema, and acute renal failure. Symptoms appear 2–3 weeks after streptococcal pharyngitis or other bacterial infection
What are the characteristic laboratory findings of postinfectious GN?
Urine sediment reveals dysmorphic RBCs or RBC casts and proteinuria (occasionally nephrotic range); serum studies are remarkable for hypocomplementemia, positive ASO titer.
What are the renal biopsy characteristics for LM, IF, EM?
LM - Glomerular hypercellularity, with epithelial and endothelial cell proliferation, and an inflammatory glomerular infiltrate composed of neutrophils. Capillary lumens are usually obliterated. Crescents may be present.
IF - Granular deposition of IgG and C3 in the glomerular tuft
EM - Large, irregular, subepithelial "hump"-shaped deposits
What is the appropriate management of postinfectious GN?
No specific therapy other than the treatment of the underlying infection in most cases. A trial of steroids may be indicated if renal failure is severe. There is no evidence supporting aggressive immunosuppression.
What is the prognosis of postinfectious GN?
Self-limited, with Cr returning to baseline in <6 weeks in most cases; hematuria typically resolves within 6 months. 15% of patients with poststreptococcal GN have persistent proteinuria at 3 years, 2% at 7–10 years. Progression to ESRD is rare, as is recurrence.
A 33-year-old man comes to the ED because of blood in his urine for 2 days. He has also been feeling unwell, with a sore throat, running nose, cough, and fever. Medical history includes three episodes of hematuria in the past that have spontaneously resolved. His temperature is 98.9°F; pulse is 82/min; respirations are 18/min, and blood pressure is 145/90 mm Hg. PE is normal. Urinalysis shows moderate numbers of erythrocytes, a few leukocytes, red cell casts, and a large amount of protein. No bacteria are cultured. A renal biopsy demonstrates large dark mesangial deposits.
IgA nephropathy
What is the typical clinical presentation of IgA nephropathy?
Gross hematuria with or without proteinuria during viral upper respiratory tract infection or GI illness, persistent microscopic hematuria, and sometimes associated dull flank pain, with a 2:1 male predominance, often presenting in the second and third decades of life
What are the most common conditions with which IgA nephropathy is associated?
Liver disease (e.g., alcoholic cirrhosis), inflammatory bowel disease, celiac sprue, and HIV
What are the characteristic laboratory findings of IgA nephropathy?
Normal or decreased renal function, persistent microhematuria, and variable proteinuria; urine sediment reveals dysmorphic RBCs and RBC casts
What are the renal biopsy characteristics of IgA nephropathy for LM, IF, EM?
LM - Focal or diffuse mesangial proliferation with matrix expansion. Crescents may or may not be present.
IF - Globular IgA deposition in the mesangium and capillary walls
EM - Electron dense mesangial deposits
What is the appropriate management of IgA nephropathy?
Most patients will benefit from ACE-I or ARB to reduce proteinuria and BP, as well as a statin. Patients with nephrotic range proteinuria may also benefit from the addition of fish oil, while patients with more severe disease require immunosuppression
What is the of IgA nephropathy of IgA nephropathy?
Patients without proteinuria and who have preserved GFR have a low risk of progressing to ESRD. Of patients who develop proteinuria, 30% will progress slowly to ESRD within 30 years
A 26-year-old man presents with hematuria, periorbital edema, and jaundice. He has a medical history of opioid use disorder with prior hospitalizations for a heroin overdose. He is on Suboxone but is non-adherent. His blood pressure is 162/102 mmHg. Physical examination is significant for scleral icterus, hepatomegaly, and palpable purpura. Serology shows decreased C3 and C4 levels and elevated anti-hepatitis C antibodies. Urinalysis demonstrates dysmorphic red blood cells and red blood cell casts
Membranoproliferative glomerulonephritis (MPGN)
What is the typical clinical presentation of MPGN?
Variable decrease in renal function, HTN, anemia, hematuria, and proteinuria
What are the most common causes of secondary MPGN?
Hepatitis B/C, cryoglobulinemia, lupus, and HIV
What are the characteristic laboratory findings of MPGN?
Variable increase in BUN/serum creatinine, anemia, low serum C3 or C4, and presence of serum C3 nephritic factor (especially in type II). Urine sediment contains dysmorphic RBCs, WBCs, and RBC casts
What are the renal biopsy characteristics of MPGN for LM, IF, EM?
LM - Thickening and "tram-tracking" (splitting) of GBM. Mesangial proliferation and interposition.
IF - Fine granular deposition of C3 and IgG deposition in the mesangium and along peripheral capillary walls. EM - Mesangial interposition and duplication of GBM. Immune complex deposits are present; the location (mesangial, subendothelial, or subepithelial) dictates the type of MPGN (I, II, or III)
What is the appropriate management of MPGN?
Treatment of the underlying disease in secondary MPGN is a mainstay of therapy. Idiopathic disease can be treated with steroids or antiplatelet agents, such as aspirin, clopidogrel, or dipyridamole. Data are limited to support the use of cytotoxic drugs
What is the prognosis of MPGN?
Complete or partial remission can be induced in secondary MPGN by the treatment of the underlying disease. In patients with idiopathic MPGN, 50%-60% will develop ESRD in 10-15 years if left untreated. How frequently does MPGN recur in the transplanted kidney and what is the outcome if it does? Type I and type III recur in ~30% of transplants
A 15-year-old boy comes to the office because of malaise, anorexia, nausea, and decreased urination. His mother says that he is having problems hearing. Physical examination shows decreased hearing bilaterally with the Rinne test and bilateral edema in the lower extremities. Urinalysis shows microscopic hematuria and proteinuria. A peripheral blood smear reveals microcytic anemia
Alport syndrome
Who is usually affected by Alport syndrome and at what age?
Usually occurs in men by 30 years of age
What is the pathogenesis of Alport syndrome?
Defects in a3, a4, or a5 chains in type IV collagen in GBM (and elsewhere)
What is the genetics of Alport syndrome?
The most common method of inheritance is X-linked, but Alport's can also be autosomal recessive, or autosomal dominant.
What is the typical clinical presentation of Alport syndrome?
Sensorineural hearing loss, ocular abnormalities (anterior lenticonus, corneal dystrophy, perimacular flecks); persistent microscopic hematuria, and renal failure
What are the characteristic laboratory findings of Alport syndrome?
Elevated BUN/serum creatinine; microscopic hematuria with dysmorphic RBCs (sometimes RBC casts)
What are the renal biopsy characteristics of Alport syndrome LM, IF, EM?
LM - More pathologic changes are seen as the patient ages. LM shows nonspecific findings: glomerular hypercellularity and lipid-laden foam cells in the interstitium
IF - Immunostaining for a chains of type IV collagen is diagnostic
EM - Initially, the GBM may be thin on EM, but over time it becomes split, frayed, and takes on a laminated appearance
What is the appropriate management of Alport syndrome?
No known treatment other than dialysis and transplantation when appropriate
Are there any postrenal transplantation issues?
Patients may develop anti-GBM disease (Goodpasture-like) because they make antibodies to the a3 and a5 chains of type IV collagen, which they lacked in their native kidneys.
A 27-year-old male presents to the clinic complaining of coughing up small amounts of blood daily for the past week. He denies smoking, sick contacts, or recent travel. Chest radiographs demonstrate interstitial pneumonia with patchy alveolar infiltrates suggestive of multiple bleeding sites. Urinalysis is positive for blood and protein. A positive result is returned for anti-glomerular basement membrane antibody (anti-GBM Ab)
Goodpasture syndrome
When do patients with Goodpasture present and who is affected?
Most common in men, ages 20-40 and 50-70 years; occurs more commonly in smokers or those with lung injury
What is the pathogenesis of Goodpasture syndrome?
Antibody formation to the a 3 chain of type IV collagen in the GBM
What is the typical clinical presentation of Goodpasture syndrome?
Malaise, rapidly progressive renal failure, anemia, hemoptysis and pulmonary hemorrhage
What are the characteristic laboratory findings of Goodpasture syndrome?
Rapidly rising BUN/serum creatinine; proteinuria (usually nonnephrotic); and positive anti-GBM antibodies, occasionally positive ANCA, and normal complement levels. Urine sediment contains dysmorphic
RBCs and RBC casts
What is the appropriate management of Goodpasture syndrome?
Apheresis, high-dose IV steroids, and cyclophosphamide
What is the prognosis of Goodpasture syndrome?
If untreated, mortality can be as high as 90%. With aggressive early treatment, prognosis improves. Both renal and patient survival mirrors the severity of disease at presentation. Relapses are uncommon.
What is the typical clinical presentation of Wegener granulomatosis?
Sinusitis, cough, dyspnea, hemoptysis, migratory pulmonary infiltrates, hematuria or tea-colored urine, proteinuria, and renal insufficiency
What are the characteristic laboratory findings of Wegener granulomatosis?
Elevated BUN and serum creatinine, positive ANCA (usually c-ANCA or anti-proteinase 3), t t ESR and CRP, hy pocomple- mentemia, urinary sediment with dysmorphic RBCs, RBC casts, and protein (usually nonnephrotic range)
What are the characteristic radiologic findings?
Sinus thickening and fluid levels on x-ray; patchy pulmonary infiltrates on CXR
What are the renal biopsy characteristics for LM, IF, EM?
LM?
Necrotizing glomerulonephritis with cellular or fibrous crescents, mononuclear interstitial infiltrate. Rarely are granulomas seen on renal biopsy.
IF?
No immunofluorescence
EM?
No immune complex deposits
What is the prognosis?
ESRD may occur in up to 25% of patients. Of patients who are dialysis dependent at presentation, 5 5%-90% may recover enough renal function to come off dialysis.
What is the appropriate management?
In patients who do not require dialysis, steroids and cytotoxic therapy (such as cyclophosphamide) is usually sufficient. If the disease is more aggressive or the patient requires dialysis at the time of presentation, plasmapheresis may be required.
What is the significance of nephrotic-range proteinuria?
It is almost invariably associated with glomerular disease
Primary glomerular diseases vs. secondary glomerular diseases?
1. Primary glomerular diseases mean that the condition occurs on its own, without another known systemic disease such as lupus or diabetes e.g. *minimal change*, *membranous nephropathy*, *focal segmental glomerulosclerosis (FSGS)*, and medication-induced glomerulopathy (e.g., NSAIDs, heroin)
2. Secondary glomerular diseases are kidney conditions with glomerular pathology in which an underlying cause can be established e.g. DM type 1 or 2, collagen vascular disease, malignancy-associated renal disease, infection-associated glomerulopathy (e.g., hepatitis B or C, HIV)
What laboratory studies may be useful in the evaluation of a patient with nephrotic syndrome?
Chemistry panel, LFTs, glycosylated hemoglobin, ANA, ANCA, serum complement studies, hepatitis panel, HIV antibodies, serum cryoglobulins, ESR, RF, SPEP, and UPEP
How is a renal biopsy useful in evaluating a patient with nephrotic syndrome?
LM, IF, and EM of glomerular tissue combined with clinical information provide a diagnosis, useful for management and prognosis.
What are the complications of the nephrotic syndrome?
Edema and ascites, skin breakdown, hypercoagulability, hyperlipidemia and accelerated atherosclerosis, immunoglobulin loss, and predisposition to bacterial infection. Heavy proteinuria itself may also accelerate the course of renal failure.
What are the most common causes of nonnephrotic proteinuria (<3 g/d)?
Hypertensive nephrosclerosis, atherosclerotic disease, chronic interstitial nephropathy, orthostatic proteinuria
Why is a renal biopsy generally not useful in evaluating patients with proteinuria of <3 g/d?
Nonnephrotic proteinuria is often associated with tubulointerstitial disease. Findings on biopsy are nonspecific and unlikely to provide enough information to make a diagnosis.
In general, what is the prognosis in patients with <3 g/d proteinuria?
The prognosis is usually good in these patients.
What is the typical clinical presentation of minimal change disease?
Edema, ascites in a child younger than 10 years; and onset over days to weeks. The distribution is bimodal, and minimal change disease can also affect elderly patients.
How common is minimal change disease?
Accounts for approximately 90% of nephrotic syndrome in children aged <10 years; accounts for approximately 20% of nephrotic syndrome in adults
What are the most common secondary causes of minimal change disease?
Lymphoproliferative disease, especially Hodgkin's lymphoma, and NSAID use
What are the characteristic laboratory findings?
Normal renal function, marked proteinuria, hyperlipidemia, hypoalbuminemia, and urinary sediment with hyaline casts and oval fat bodies
What are the renal biopsy characteristics for LM? Normal IF? Negative EM?
LM - Normal
Normal IF - Negative
EM - Effacement of foot processes
What is the appropriate management?
Prednisone 1 mg/kg (up to 80 mg every day) × 4-8 weeks; gradually taper if a response is noted. ACE-Is may also be used as an adjunct to therapy (to reduce proteinuria), or as sole therapy in mild cases.
What is the prognosis?
90% of children and 60% of adults respond well to prednisone. If associated with lymphoma, proteinuria decreases if lymphoma is in remission.
What is the relapse rate?
~75% may relapse and require another course of treatment. 25%-30% may relapse frequently or be resistant to treatment.
What treatment should be considered in frequent relapsers?
Prolonged treatment with immunosuppressants chlorambucil, cyclosporine, or cyclophosphamide should be considered. Mycophenolate mofetil has also been studied for this disease, with favorable results.
What are the complications of minimal change disease?
Peritonitis in children (especially Streptococcus pneumoniae); AKI (generally reversible), side effects of long-term steroid therapy (osteopenia/-porosis, Cushing syndrome)
What is the typical clinical presentation of membranous nephropathy (MN)?
Most patients present with slow-onset nephrotic syndrome; others may be diagnosed with asymptomatic proteinuria. BP may be normal or elevated.
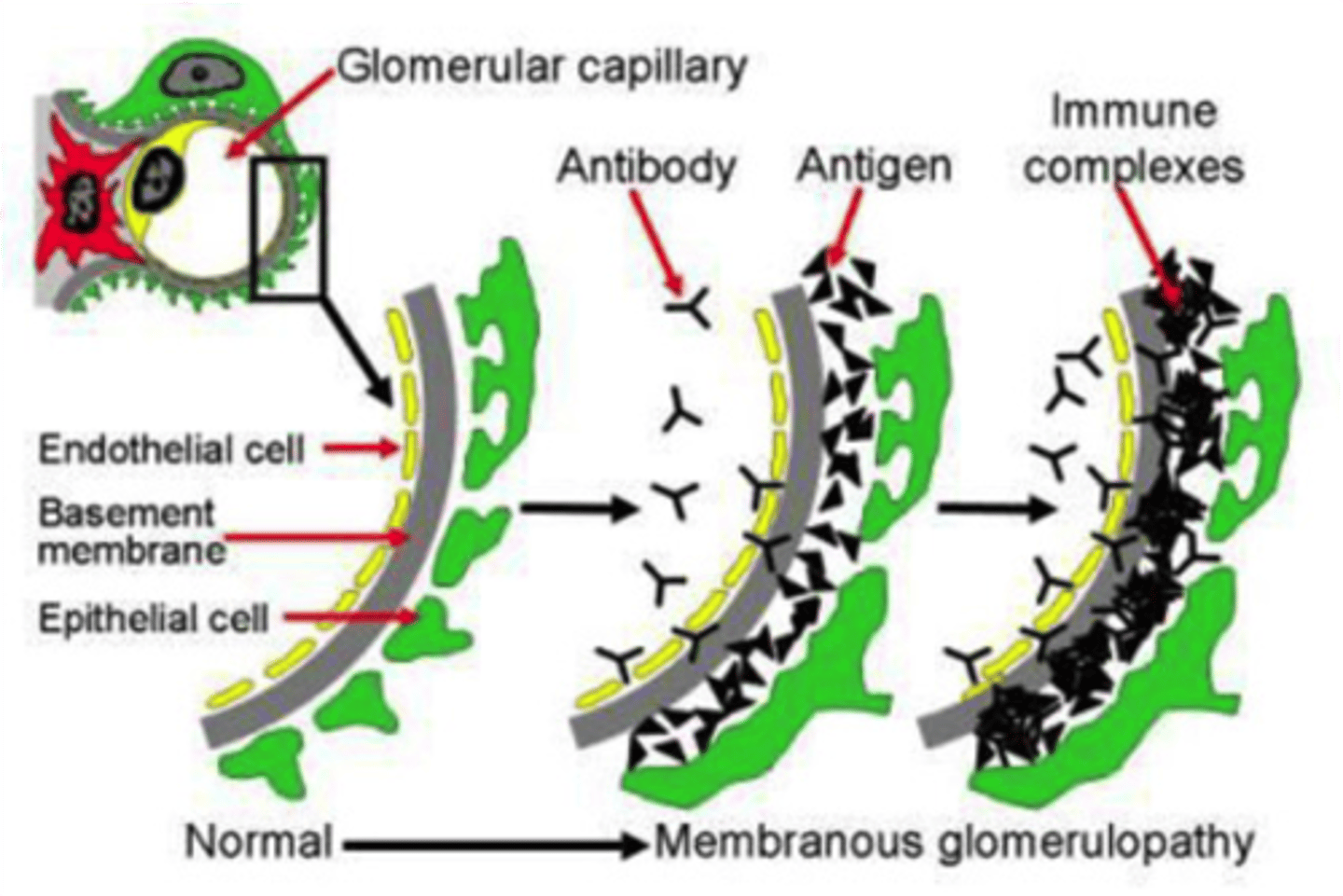
How common is secondary MN?
Most common cause of nephrotic syndrome in adults (accounts for 15%-20%). Secondary causes can be identified in ~30% of patients with biopsy-proven MN.
What are the most common causes?
Malignancy (~10%), infection (e.g., hepatitis B/C, syphilis, leprosy), lupus and other connective tissue diseases, gold therapy, penicillamine, and sickle cell disease
Which malignancies are common in secondary MN?
Solid organ tumors such as lung, colon, kidney, and breast
What is the pathogenesis of MN?
MN is caused by immune complex formation in the glomerulus. The immune complexes are formed by binding of antibodies to antigens in the glomerular basement membrane. The immune complex serves as an activator that triggers a response from the C5b - C9 complements, which form a membrane attack complex (MAC) on the glomerular epithelial cells. This, in turn, stimulates release of proteases and oxidants by the mesangial and epithelial cells, damaging the capillary walls and causing them to become "leaky".
What are the characteristic laboratory findings?
Marked proteinuria, hypoalbuminemia, hyperlipidemia; renal function may be normal or decreased; urinalysis reveals glucosuria, oval fat bodies, fatty casts, lipid droplets, and microscopic hematuria (in about 50% of cases). RBC casts are rare.
What are the renal biopsy characteristics for LM?
Thickened GBM
IF?
Diffuse granular pattern of IgG and C3 deposits along the GBM. Deposits of IgG may also be seen along the tubular basement membrane with secondary MN.
EM?
Small, uniform subepithelial deposits in idiopathic MN.
In secondary MN, immune complex deposits can be seen in the mesangium and in the subendothelial space.
In MN associated with lupus, tubuloreticular structures in the endothelial cells are seen. Effacement of foot processes is common in all types of MN, as is GBM expansion.
What is the appropriate management?
Depends on the risk of progression to ESRD; judged by the amount of proteinuria and the degree of renal insufficiency. Patients at moderate to high risk should be treated with a combination of glucocorticoids and cytotoxic therapy (cyclophosphamide). Those at low risk can be treated with ACE-Is. Lipid-lowering agents should be used in cases of persistent nephrotic syndrome.
What is the prognosis?
25% undergo spontaneous remission in 5 years, 25% undergo spontaneous partial remission in 5 years, 25% have persistent nephrotic syndrome with stable or slowly deteriorating renal function; and 25% develop ESRD in 20-30 years.
What is the typical clinical presentation of FSGS?
Edema, HTN, decreased renal function to varying degrees; most common cause of nephrotic syndrome in African Americans, especially young males
What are the most common causes of secondary FSGS?
Reflux nephropathy, massive obesity, heroin use, HIV infection, and congenital renal aplasia
What are the characteristic laboratory findings?
Marked proteinuria, hypoalbuminemia, hyperlipidemia; renal function may be normal or decreased; urinalysis reveals oval fat bodies, fatty casts, and lipid droplets. Microscopic hematuria may be present, but gross hematuria or RBC casts are rare.
What are the renal biopsy characteristics for LM?
Presence in some, but not all, glomeruli segmental areas of mesangial collapse and sclerosis. There are several variant morphologies, which have an impact on prognosis. It is important to remember that FSGS may not be seen on biopsy because of sampling error.
What are the renal biopsy characteristics for IF?
There may be nonspecific binding of IgM and complement to sclerotic areas. There is no particular "classic" IF pattern for FSGS.
What are the renal biopsy characteristics for EM?
Fusion of foot processes
What is the appropriate management?
ACE-I should be used for reduction of proteinuria. Immunosuppression with prednisone is the first-line therapy. Steroid-resistant cases may benefit from addition of cyclosporine. Relapses require reinitiation of steroids.
What is the prognosis?
Prednisone induces complete or partial remission in 40%-80% of patients with FSGS but requires a prolonged course. Gradual progression to ESRD over 5-12 years may be expected, especially in patients who did not initially respond to prednisone or have had multiple relapses. Approximately 30% experience recurrence of disease in a transplanted kidney.
A 32-year-old female presents with fever, chills, nausea, and flank pain for 24 hours. She developed dysuria and urinary frequency 3 days prior and states that both have worsened. On physical exam, you note suprapubic abdominal pain and CVA tenderness. The urinalysis reveals white blood cell casts. What's the Dx?
Pyelonephritis
What is pyelonephritis?
Inflammation of the kidney due to a bacterial infection.
● The inflammation of the kidney is due to a specific type of urinary tract infection (UTI). The UTI usually begins in the urethra or bladder and travels to the kidneys.
What is the most common bacterial cause of acute pyelonephritis?
Escherichia coli is the most common bacterial cause of acute pyelonephritis.
Describe the common presentation of a patient with acute pyelonephritis?
Patients will commonly present with fever, chills, nausea, and flank pain
What type of cell casts are a classic feature in acute pyelonephritis?
White blood cell casts are a classic feature in acute pyelonephritis.
When are IV antibiotics indicated for the treatment of acute pyelonephritis?
IV antibiotics are indicated for inpatients who are toxic or unable to tolerate oral antibiotics
What is the first line of treatment in outpatients with pyelonephritis (antibiotic class)?
The first line of treatment in outpatients with pyelonephritis is (antibiotic class) fluoroquinolones
Describe the management of acute pyelonephritis in pregnant women
Management of acute pyelonephritis in pregnant women includes hospital admission for parenteral antibiotics. Empiric therapy includes IV/IM ceftriaxone
A renal disorder defined as interstitial fibrosis and atrophy of tubules?
Chronic pyelonephritis is a renal disorder defined as interstitial fibrosis and atrophy of tubules.
What is the most common cause of chronic pyelonephritis in adults?
The most common cause of chronic pyelonephritis in adults is chronic urinary tract obstruction.
Patients with pyelonephritis usually have what type of pain?
Patients with pyelonephritis usually have flank pain due to inflammation sensitizing the nerves of the renal capsule
What is acute kidney injury (AKI)?
Acute kidney injury is a rapid decrease in renal function over days to weeks, causing an accumulation of nitrogenous products in the blood (azotemia) with or without a reduction in the amount of urine output
What defines Acute Kidney Injury?
- Increase in the serum creatinine value of ≥ 0.3 mg/dL (26.52 micromol/L) in 48 hours
OR
- Increase in serum creatinine of ≥ 1.5 times baseline within the prior 7 days
OR
- Urine volume < 0.5 mL/kg/hour for 6 hours
Define Azotemia
Retention of nitrogenous wastes (from metabolic processes which produce nitrogen) causing elevated BUN and creatinine
Define uremia
Uremia is the term for high levels of urea in the blood
● Urea is one of the primary components of urine. It can be defined as an excess of amino acid and protein metabolism end products, such as urea and creatinine, in the blood that would be normally excreted in the urine
Define proteinuria
Protein excretion in urine > 150 mg/24hrs, >3.5 g=nephrotic range
Define euvolemic
Normal blood volume in body
Define creatinine
Product of muscle metabolism, produced at a relatively constant rate, cleared by renal excretion, filtered by the glomerulus, and not reabsorbed
Define Cystatin C
Endogenous marker of GFR, freely filtered at glomerulus, less dependent on muscle mass, reabsorbed and partially metabolized in renal tubular cells
Define BUN (blood urea nitrogen)
Synthesized mainly in liver, end product of protein catabolism - It's carried in your blood, filtered out by your kidneys, and removed from your body in your urine