Quiz #1 - The Stratosphere & Its Pollution
1/71
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
72 Terms
planetary accretion
physical properties brought particles together forming planetesimals
gravitational pull attracts additional objects, forming a protoplanet
proto-Earth continues growing over a very long period of time
protoplanet collision
small protoplanet Theia collides into the young proto-Earth
protoplanet Theia mixed with proto-Earth while some debris was held in orbit
disk of debris from Theia and proto-Earth aggregates to form the Moon
asteroid bombardment
asteroids and comets struck the Earth and Moon (as well as other planets in the inner solar system)
constant bombardment caused the Earth to remain molten with episodes of volcanic eruptions
stratification & cooling
materials comprising the Earth begin to separate with respect to density
denser materials sank to the core
lighter materials formed moved to the outer reaches of the Earth
Earth begins to cool
stratification
layering is found in the abiotic (non-living ) spheres of the Earth
density (d) = mass (m) / volume (v)
mass= amount of matter comprising an object
matter=the stuff that makes up everything
volume=the amount of space an object occupies
atom
the basic building block of matter comprised of subatomic particles including proton(s), neutron(s), and electron(s)
three subatomic particles
proton (p): positively charged particle
electron (e-): negatively charged particle
neutrons (n): neutral particles
nucleus
positively charged center of an atom comprised of proton(s) and neutron(s)
orbitals
sites where electrons (e-) could be
cation
positively charged atoms or molecules
anion
negatively charged atoms or molecules
dipole
molecule with positive and negative charges; partial charges on molecules
volume
the amount of space that a substance occupies
solid: definite
liquid: definite
gas: indefinite
shape
the structure or form that a substance occupies in 3D space
solid: definite
liquid: indefinite
gas: definite
intermolecular forces
the electrostatic forces between molecules usually due to charged or partially charged species
solid: strong
liquid: moderate
gas: weak
intramolecular forces
forces within the molecule keeping it together, specifically the bonds between the atoms
density
a measure of compactness or closeness of the molecules, atoms, etc.
solid: high
liquid: moderate
gas: low
compressibility
ability to be reduced in volume
solid: incompressible
liquid: incompressible
gas: compressible
particle motion
movement of the molecules, atoms, etc.
solid: low
liquid: moderate
gas: high
kinetic energy
energy of a substance due to motion
solid: low
liquid: moderate
gas: high
geosphere (1/4)
the solid Earth
“geo” means earth
outer layers solidified, the crust began to form while maintaining molten layers below
large cracks created plates and landmasses (eventually creating the continents we know today)
all metals, rocks, minerals, soils, landforms, etc. from the core to the crust
solid and liquid particles comprising Earth itself
atmosphere (2/4)
“atmo” means air
the air above Earth’s surface
early atmosphere consisted of gases from volcanic eruptions: water vapor (H2O(g)) and carbon dioxide (CO2)
as the biosphere evolved, carbon dioxide (CO2) was converted to oxygen (O2)
mixtures of gaseous particles that surround Earth
no definite shape nor volume as it flows into space
protects life from incoming radiation
provides warming effect to maintain life
has undergone and will continue to undergo changes
gases found in the outer edges slowly leave Earth's atmosphere into outer space (less massive)
the molecules within this sphere will probably not collide
as altitude increases, gravity and molecules decrease; less particles, less collisions, less reactions
as altitude decreases, gravity and molecules increase; more particles, more collisions, more reactions
other factors, such as temperature & water vapor, are important to atmospheric pressure
hydrosphere (3/4)
“hydro” means water
all water on Earth’s surface
comets and asteroids brought water (H2O)
volcanic eruptions released water vapor (H2O(g)) into the atmosphere
as the Earth cooled, water (H2O(g)) began to condense forming bodies of water (H2O(l)) and eventually ice (H2O(s))
solid=snow/ice
liquid=water
properties of water are crucial for life on Earth
freshwater = 2.5% of total global water
biosphere (4/4)
“bio” means life
the living things on Earth
organisms that could survive without oxygen (O2) were first to appear on Earth
some organisms evolved photosynthetic abilities, producing oxygen (O2)
change in the atmosphere supported new life forms that relied on oxygen (O2)
all biotic (living) organisms, such as plants, animals, fungi, and microorganisms, on Earth
have self-sustaining processes or functions to external stimuli
ability to grow, reproduce, move, metabolize, breathe, excrete, etc.
lithosphere
part of the lithosphere
“litho” means stone
all metals, rocks, minerals, soils, landforms, etc. from the crust to the upper mantle
rigid and brittle
mostly solid until seismic triggers force magma towards the surface as lava
provides important minerals (serving as nutrients) for plants and animals
provides resources (metals, fossil fuels, ores, etc.) needed for human activities
provides habitats/homes
cryosphere
“cryo” means icy cold or frost
all water on Earth in its solid form (H2O(s))
different parts of the cryosphere experience changes on different timescales
exosphere
temperature is independent of altitude & varies with solar activity
thermosphere
higher temperature (hotter) with increasing altitude
mesosphere
lower temperature (colder) with increasing altitude
stratosphere
“strat” means layer
higher temperature (hotter) with increasing altitude
lacks the turbulence and updrafts from troposphere
troposphere
“tropos” means change
lower temperature (colder) with increasing altitude
75% of the mass of the entire atmosphere
warmer air below will rise and cool air above will sink
99% of all water vapor ( H2O(g) ) in the atmosphere is found in the troposphere
3 main circulations between due to the Earth's rotation: Hadley Cells (closest to equator), Ferrel Cells (middle cells), Polar Cells (closest to poles)
Earth in & out of balance
equilibrium: a state of balance in which external and/or internal influences cancel each other to maintain that same state
systems maintaining the balance can be static (remaining the same over long periods of time) or dynamic (constantly changing)
humanity disturbs the exchange, impacting the other spheres, as well as other living organisms within the biosphere itself
technosphere
all technological objects (machines, factories, cars, buildings, internet, AI, agriculture, etc.) made or modified by humans for human activities and/or habits; the built environment
“biosphere is extremely good at recycling the material it is made of … the technosphere, by contrast, is poor at recycling” – UNESCO 2024
primary source of modern pollution
atmospheric chemistry
understanding the composition of the atmosphere and relevant photochemical reactions before applying the science to the stratosphere
heterogeneous
no uniformity in the composition
homogeneous
uniform composition throughout
main atmospheric gases
Nitrogen (N2)
Oxygen (O2)
Argon (Ar) (inert/noble gas; do not react with other molecules)
trace atmospheric gases
very low concentration but still has significant impact on the planet
Carbon Dioxide (CO2)
Ozone (O3)
Methane (CH4)
Nitrous Oxide (N2O)
Water Vapor (H2O(g))
Neon (Ne) (inert/noble gas; do not react with other molecules)
Helium (He) (inert/noble gas; do not react with other molecules)
Krypton (Kr) (inert/noble gas; do not react with other molecules)
Xenon (Xe) (inert/noble gas; do not react with other molecules)
photochemistry
chemical reactions and/or physical processes that occur when light interacts with a molecule
when photons excite molecules to higher energy (vibrational and/or electronic) levels, leading to chemical transformations
transformations can include chemical reactions, rearrangements, or emission of energy as heat or light
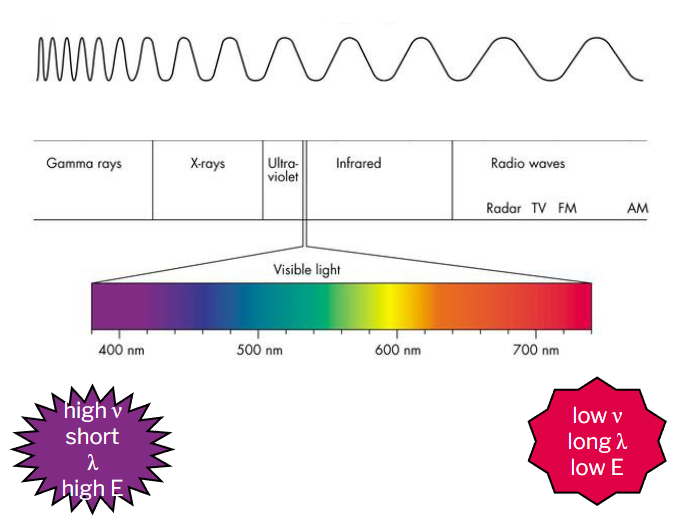
electromagnetic spectrum
the range of electromagnetic radiation (electric and magnetic fields) from the sun
the particles that make up electromagnetic radiation are referred to as photons or “energy packets”; they exhibit particle-wave duality
wavelength (λ) is the distance from peak to peak
frequency (ν) is the number of waves that pass through a point in a second
increasing frequency from right to left (700nm=low v, long λ, low E to 400nm=high v, short λ, high E)
atmospheric photochemistry
the study of gaseous molecules that react with “light” within the atmosphere
M + photon → M* → Ʌ• •Ʌ (where “M” represents a molecule and “*” denotes an excited state for M) { vibrational relaxation, fluorescence, photodissociation }
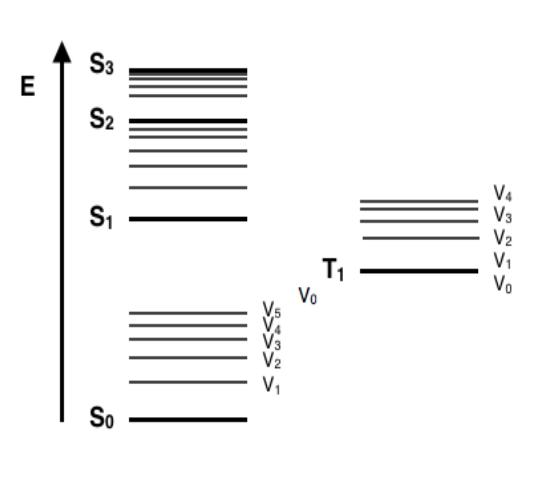
Jablonski Energy Diagram
a diagram that shows the electronic states of a molecule and the various radiative (light-emitting) and non-radiative (heat-releasing) transitions between them
shows how a molecule returns to its ground state after absorbing electromagnetic radiation (light)
only two electrons can occupy each orbital
singlet ground state (S0): most stable, lowest-energy electronic state of a molecule where all electrons are paired
singlet excited state (S1…): promoted electron has the same spin orientation as it had in the ground state (paired)
triplet excited state (T1…): promoted electron has the same spin orientation (parallel) as the other unpaired electron
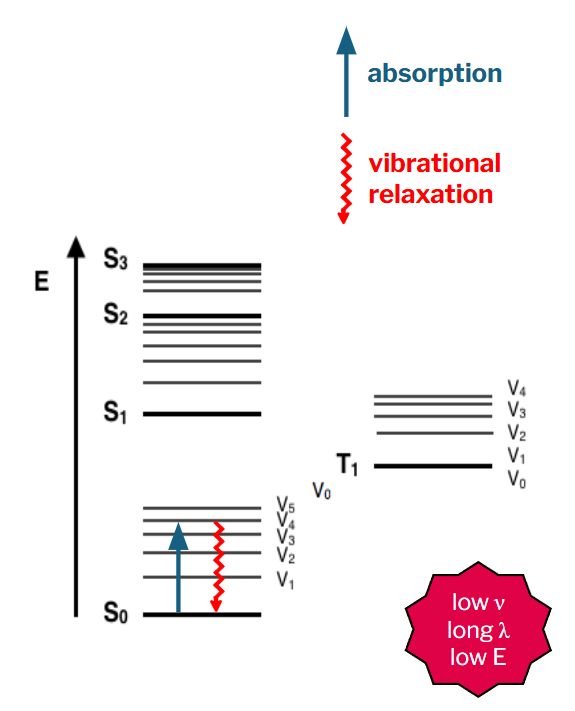
vibrational relaxation
photon with low energy can cause the greenhouse effect
traps heat, specifically the sun’s radiation, in a planet’s lower atmosphere
greenhouse gases (GHGs) prevent the Earth from losing heat to space
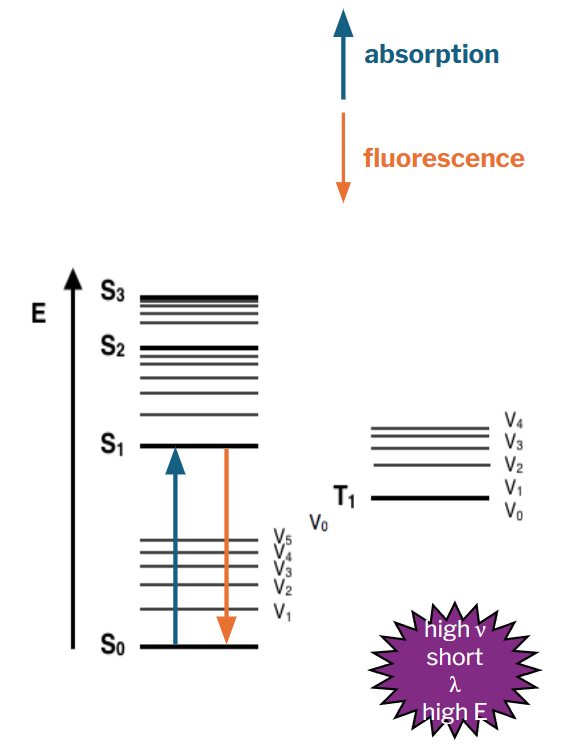
fluorescence
the right amount of energy causes electrons to jump from a normal, low energy “ground state” to an unstable higher energy “excited state”
molecule cannot remain in an unstable state for long
electrons immediately falls to its ground state giving off light
photodissociation
photon with high enough energy can break chemical bonds within the molecule
creating radicals (highly reactive fragments) that can then undergo further reactions
can lead to chain reactions
M + photon → M* → Ʌ• •Ʌ; ex. A-B (molecule = M) + photon → A-B* → A• + B•
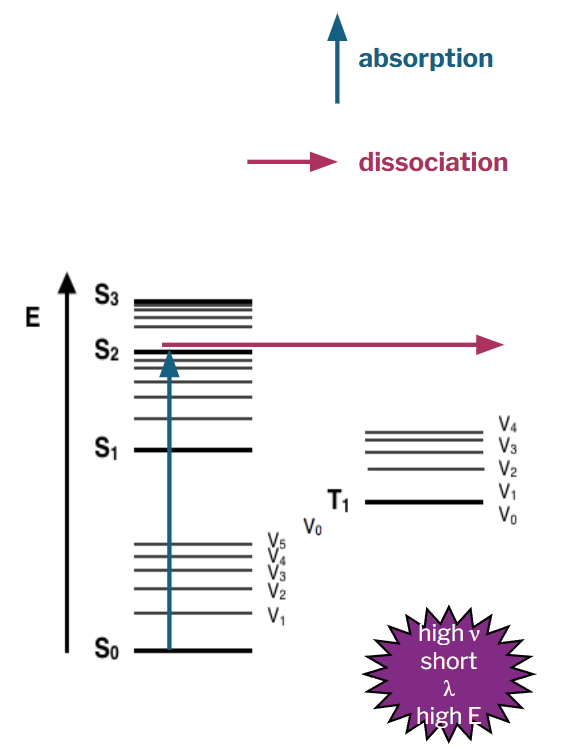
applying atmospheric chemistry
using what we have learned about the relevant reactions, we will now look at photodissociation in the stratosphere and understand its importance
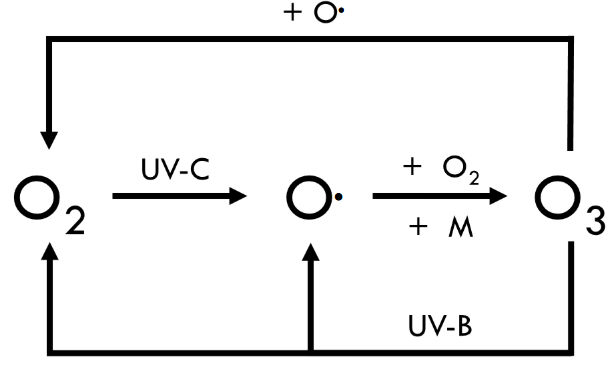
oxygen photodissociation
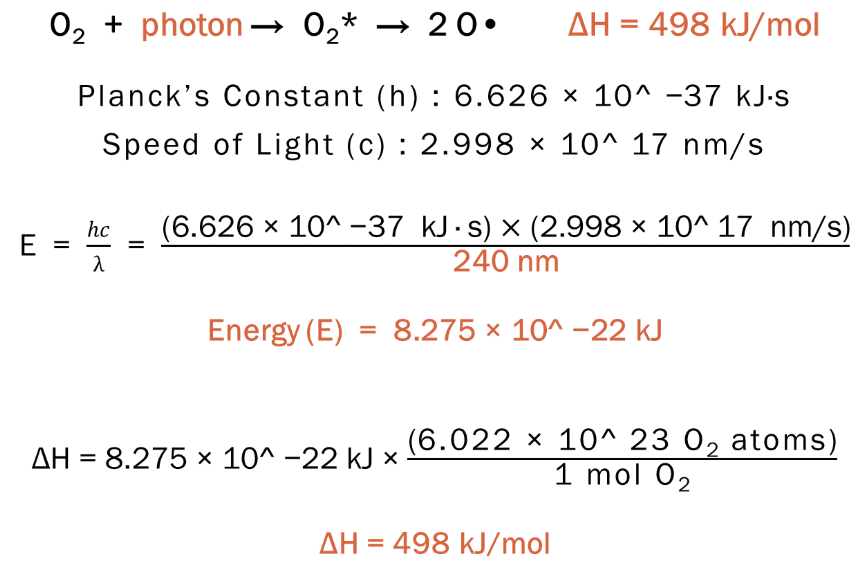
O2 + photon (λ ≤ 240 nm) → O2* → 2 O•
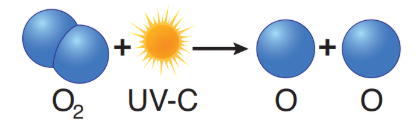
ozone formation
O• + O2 → O3 + heat
O• + O2 + M → O3 + M + heat
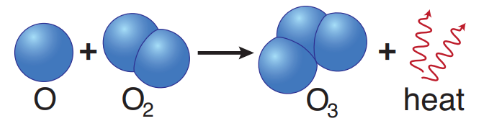
oxygen recombination
O• + O• → O2
however, two oxygen atoms (O•) can recombine to form a molecule of oxygen (O2)
importance of ozone (O3)
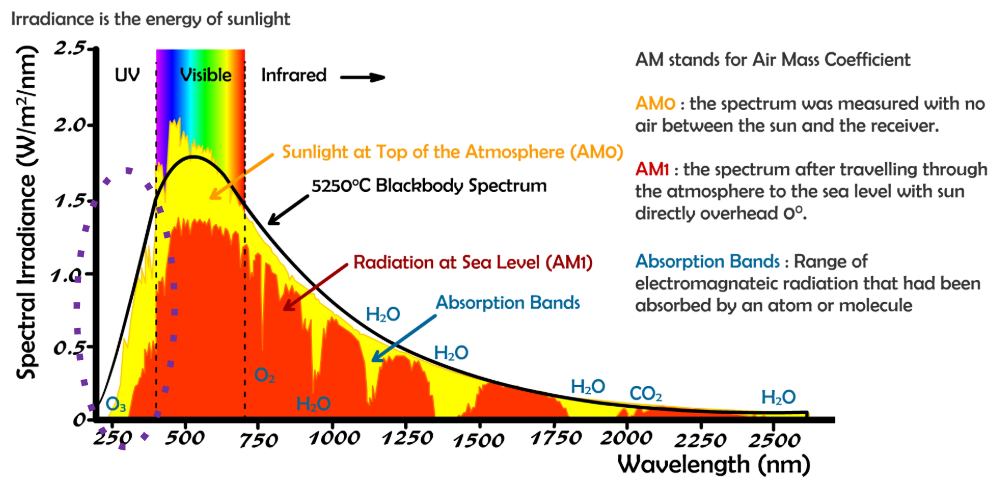
absorption spectrum = a plot of wavelength versus the extent of absorption
“obtained by measuring the amount of electromagnetic radiation absorbed by a sample at various wavelengths” – Advances in Nanotech, 2022
high energy photons that do reach the earth are caught in the stratosphere
ozone (O3) filters most of the ultraviolet (UV) range
solar radiation spectrum
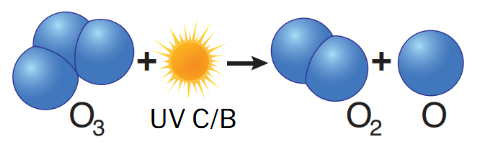
ozone photodissociation
O3 + photon (λ ≤ 320 nm) → O2 + O•
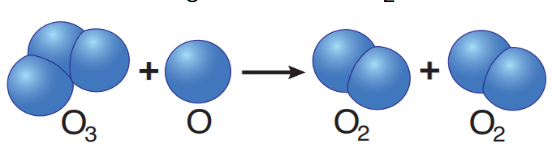
ozone conversion
O3 + O• → 2 O2
collisions with insufficient energy to result in a reaction
Chapman Cycle
the photochemical reactions conducted in the stratosphere that describe the formation and destruction of ozone (O3)
molecular oxygen reactivity
a photon with a wavelength of 240 nm has the right amount of energy (E) to excite oxygen (O2) which then breaks apart to monoatomic oxygen radical (O•)
radical: unpaired valence electron
ozone layer
ozone (O3) in the stratosphere that protects life on earth from ultraviolet (UV) radiation
not a “layer” since the gas is dispersed throughout stratosphere
a region in the stratosphere with higher concentration (or presence) of ozone (O3)
ozone holes
thinning of the ozone (O3) layer in the stratosphere, typically found above the North and/or South Pole(s)
less than 200 Dobson Units (DU)
average ozone globally: 300 DU; anthropogenic ozone loss: < 200 DU; average ozone hole: 100 DU
1 DU = 0.01 mm thickness of ozone (O3) at STP if compressed into a layer at sea level.
when ozone holes occur
Antarctic (South Pole): September to November; first observed 1979; ideal conditions makes it larger
Arctic (North Pole): January to March; more of an ozone “dip”; first ozone hole appeared in 2011
generally, ozone (O3) holes have recovered but still exist and relevant reactions must be considered
ozone depleting substances (ODSs)
cfcs, hcfcs, and halons
NAMING HALONS
1st = # of C atoms
2nd = # of F atoms
3rd = # of Cl atoms
4th = # of Br atoms
ex. CBrF3 = halon-1301; CBrClF2 = halon-1211
products containing ODSs
refrigerators, air conditioners, foam, aerosol propellants, fire suppressant, chemical manufacturing, solvent in degreaser lubricants, paint stripping, pesticide
catalysts destroying ozone
radical species of chlorine (Cl•) and bromine (Br•) broken off from ozone-depleting substances (ODSs)
chlorine (Cl•) and bromine (Br•) radicals are the products from the interaction of ultraviolet (UV) radiation with ODSs within the stratosphere
CFCl₃ + photon → CFCl₂• + Cl•
simplifying the radicals
X = Cl• or Br•
X' = the “partner” halogen radical in a reaction
if X is Cl•, then X’ is Br•, and vice versa
deactivating the radicals
chlorine (Cl•) and bromine (Br•) radicals could react with other molecules in the stratosphere resulting in deactivation of the reactive species
reservoir species are relatively stable, non-radical compounds that temporarily store radical chlorine (Cl•) and bromine (Br•) preventing them from destroying ozone (O3)
these reservoirs act like “holding tanks” for radicals until released under the right conditions
X and X’ will be used to represent chlorine (Cl•) or bromine (Br•) radicals trapped within the reservoir species… when “X” is bromine (Br) … hypobromous acid (HOBr) = HOX; bromine nitrate (BrONO2) = XONO2; hydrogen bromide (HBr) = HX
activating the catalyst
cold temperature +
North & South Poles; the South Pole has the colder temperatures; hence, the size difference
no “light” +
stops light-driven atmospheric reactions; therefore, inactive molecules accumulate
pressure drop +
creates a vortex; reservoir species are trapped in an area
= polar stratospheric clouds
tiny ice crystals that “catch” the inactive molecules; H2O(g) → H2O(s)
polar stratospheric clouds (PSCs)
polar Stratospheric Clouds (PSCs) give the inactive molecules surfaces to become active molecules for ozone (O3) destruction
Cl₂, Br₂, and BrCl are released as gases
when ultraviolet (UV) light returns, these molecules photodissociate:
Cl2 + hv → 2 Cl•
Br2 + hv → 2 Br•
BrCl + hv → Br• + Cl•
catalytic ozone destruction: Mechanism 1
occurs when [O•] is high (upper stratosphere)
in the upper stratosphere there is enough high-energy UV-C radiation that can effectively photodissociate molecular oxygen (O₂), generating a high concentration of monoatomic oxygen radicals (O•)
reaction #1: X• + O3 → XO• + O2
reaction #2: XO• + O• → X• + O2
O3 + X• + XO• + O• → XO• + O2 + X• + O2
overall reaction: O3 + O• → O2 + O2 = O3 + O• → 2 O2
X• is recycled; therefore, it is a catalyst for ozone (O3) destruction
catalytic ozone destruction: Mechanism 2
occurs when [O•] is low (lower stratosphere)
by the time solar radiation penetrates down into the lower stratosphere, most of the high-energy UV-C photons capable of splitting molecular oxygen (O₂) have already been absorbed at higher altitudes
reaction #1: X• + O3→ XO• + O2
reaction #2: X’• + O3 → X’O• + O2
reaction #3: XO• + X’O• → → X• + X’• + O2
2 O3 + X• + X’• + XO• + X’O• → XO• + X’O• + X• + X’• + 3 O2
2 O3 + X• + X’• + XO• + X’O• → XO• + X’O• + X• + X’• + 3 O2
overall reaction: 2 O3 → 3 O2
X• is recycled; therefore, it is a catalyst for ozone (O3) destruction
a world avoided
between 1970 and 1980, scientists discovered that chlorofluorocarbons (CFCs) and related chemicals destroyed ozone (O3)
in 1987, the Montreal Protocol, an international treaty was designed to protect the ozone (O3) layer by phasing out the production and consumption of ozone-depleting substances (ODSs)
the protocol went into effect in 1989, after being ratified by all 198 United Nations (UN) member states
the ban on ODSs
developed countries – stricter/earlier phaseout deadlines
developing countries – delayed phaseout timelines to allow for economic adjustment
scope: developed countries under Montreal Protocol; source: EPA or UNEP
while this is critical for protecting the ozone (O3) layer, their replacements, hydrofluorocarbons (HFCs) do not harm ozone (O3) but are powerful greenhouse gases
other catalysts for ozone holes
chlorine (Cl•) and bromine (Br•) radicals but also, radicals of…nitrogen oxides (NOx); nitrogen monoxide radical (NO•); nitrogen dioxide radical (NO2•) and hydrogen oxides (HOx); hydroxyl radical (HO•); hydroperoxyl radical (HO2•)
NOₓ and HOₓ are mostly made in the troposphere, and only a fraction of these radicals or their precursors is transported into the stratosphere.
why it matters
Why should we be concerned about stratospheric ozone (O3) if ozone-depleting substances (ODSs) are banned and the other catalysts (NOx and HOx) are only transported in fractions from the troposphere?
rockets (and other high-altitude combustion sources) directly inject nitrogen oxides (NOx) into the stratosphere, bypassing slow transport
even small amounts of nitrogen oxides (NOx) can participate in catalytic ozone (O3) destruction cycles, locally depleting ozone (O3)
human activities can still influence stratospheric chemistry, even after reducing long-lived pollutants like chlorofluorocarbons (CFCs)
rockets & nitrogen oxides (NOx)
high-temperature combustion of rocket fuels produces nitrogen monoxide (NO•) via thermal excitation
temperatures are high enough to break the strong triple bond in molecular nitrogen (N2), and double bond in molecular oxygen (O2)
nitrogen monoxide radical (NO•) can react with ozone (O3) via Mechanism 1 or 2
these reactions catalytically destroy ozone