DO MCM 03 Protein Structure and Function
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
What is transcription? What is Translation?
Transcription: DNA —> RNA; Translation: RNA —→ Protein
Which Amino Acid can’t be coded by the genetic code alone?
Selenocystein: Needs the genetic code and secondary structure in the mRNA
What is the zwitterion form? When does it occur?
It is when the protein’s amino group is Protonated and the Carboxyl group is Deprotonated. It occurs at physiological pH (7.4)
what is protonation/deprotonation dependent on?
pH and pKa
What happens when pH = pKa?
groups is protonated in half of the molecules and is deprotonated in the other half.
What are the hydrophobic Amino acids?
glycine, Alanine, Proline, Valine, Leucine, Isoleucine, Phenylalanine, Tyrosine, Tryptophan, Methionine
What force is responsible for hydrophobic cores made up of hydrophobic Amino Acid?
Van der Waals Forces
What are the Polar, Uncharged Amino Acid?
Asparagine, Glutamine, Serine, Threoine, Cysteine, Selenocystein
dksl is a protein with strong antioxiant activity. Which amino acid do you expect to be in abunance?
Selenocysteine
Which group of amino acids uses hydrogen bonding interactions?
Polar, Uncharged Amino Acids
Which amino acids create disulfide bridges? How does this affect the protein?
cysteine. Increases stabilization
How is Cystine formed?
Formed via disulfide bridge between two cystEine, creating a dimmer. This is formed under oxidative conditions!
What are the charged Amino Acids? How do they interact with each other/ other charged molecules?
Aspartate, Glutamate, Arginine, Lysine, Histidine;
interact via ionic bonds
What are peptide bonds? Where are they formed?
Peptide bonds are the bonds that hold individual amino acids together. They are formed between the amino group/carboxyl group of different amino acids.
If somebody’s DNA got damaged, which level of structure is affected in proteins?
Primary Structure because the genetic code determines this
how many free amino/carboxyl groups are there in a protein?
1 each
Describe the four levels of protein structure
Primary: protein sequence
Secondary: localied substructure in proteins
Tertiary: 3d structure of protein
Quarternary structure: association between polypeptide chains
what are the four main types of secondary structures? What bond holds them together?
Alpha helixes, Beta sheets, random coils, Beta turns. H-bonds hold them together.
You noticed that a patient has aggregation of a specific protein. What secondary structure is most likely in excess?
Beta Sheets
What are the four super-secondary structures?
Helix-turn helix, Helix-loop-helix, Leucine Zipper, Zinc Finger
Bam! a source of radiation completely destroyed all super-secondary structures in a person. What genetic function would be the most impaired? Why?
Transcription. Because these super secondary structures are often used as DNA-binding zones for transcription factors.
a witch cursed you! now, every 7th amino acid is mutated. Which protein structure will be the most affected?
Leucine Zipper, because every 7th amino acid in the zipper is a leucine.
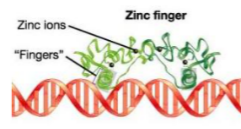
What is the purpose of Zinc Finger?
Necessary for stabilization.

What is this? Label
What is this? Label?
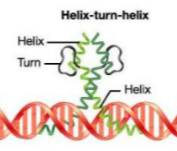
What is this? Label?
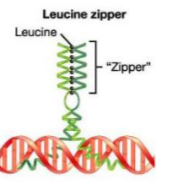
Which structure is IMPORTANT for protein function and why? Give examples
Tertiary Structure because these 3d structures creates pockets that is important for function! For examples, Myoglobin uses pockets to bind Oxygen and Enzymes uses these pockets as active sites.
What are protein domains? (T/F) They have similar functions
protein regions that folded independently; False, they can have different functions within the protein
what are some examples of different domains found in a protein?
a protein could have a ligand-binding domain, transmembrane domain, and a cytoplasmic domain.
a protein has a trans membrane domain. describe the amino acid/structure found in this domain.
hydrophobic Amino Acids, mainly alpha helixes
Describe the chains of Type II collagen
Homotrimer; three identical chains held together by non-covalent interactions/ covalent bonds
Describe the chains of heterotrimeric G-Proteins
Heterotrimer; 3 different protein chains (alpha, beta, gamma); held together by non-covalent interactions
Describe the chains of hemoglobin
2 alpha chains and 2 beta chains. held together by noncovalent interactions (A2B2 tetramers)
what are the advantages of having quaternary structure? Examples of each?
Increased cooperative (Hemoglobin),stability (collagen), and regulate activity of protein (heterotrimeric G proteins)
What determines how a protein folds?
Primary structure
What are chaperonins?
accessory proteins that some proteins require to fold to their native forms
Describe the difference between HSP70 and HSP60
70: bind to the growing protein and prevent it from folding too early
60: Folds the protein using ATP by using a template
What two things does protein misfolding lead to?
aggregation/precipitation —→ pathological consequence
What are the four main causes of protein misfolding
Mutation, proteolytic cleavage, oxidation, environmental conditions
List the symptoms of a1- antitrypsin deficiency as a result of mutation.
aggreagation of mutant protein in ER of liver, liver dysfunction (child)/cirrhosis (adults), lungs problems due to lower antitrypsin in circulation
what is the function of a1-antitrypsin, where produced, secreted?
protease inhibitor, produced in liver, secreted in circulation.
what are the three forms/causes of creutzfeld-jakob disease. What are the percentages of each forms?
sporadic form due to unknown cause (85%)
familail form due to mutation (5)
acquired from abnormal protein from another source (mad cow)
why does abnormal structure caused by creutzfeld-jakob increase exponentially over time?
This is because the abnormal structure could induce change in the normal type to become like the abnormal structure. Thus, it is capable of creating more of themselves.
What is creutzfeld-jakob? What could this disease lead to?
denaturation of prion proteins. These proteins will precipitate in the brain, causing fast brain degeneration/eath
How does AA amyloidosis occur? What is this associated with?
precipitation of serum amyloid A Fragments due to proteolytic cleavage; rheumatoid arthritis
How does Al amyloidosis occur? What is this associated with?
precipitation of antibody Light chain fragments (bence-jones proteins) due to proteolytic cleavage; multiple myeloma (cancer of antibody-producing plasma cells)
How does Alzheimer disease occur? What is this associated with?
AB amyloidosis. Caused by precipitation of B-amyloid protein which is the precursor for the amyloid protein. Causes Brain degeneration
Why are heinz body formed due to G6PD deficiency?
G6PD makes NADPH which protects against oxidative damage. A deficiency in this enzyme would make the Hb in RBC more prone to this damage. The result is heinz bodies
list the environmental conditions that would cause protein denaturation
Extreme pH, high temperature, exposure to organic solvents
Give an example of mutation leading to beneficial changes in protein structure.
In insulin, exchanging Pro28 and Lys29 leads to less insulin aggregation, better absorption and faster action.