Oxygen dissociation curve
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
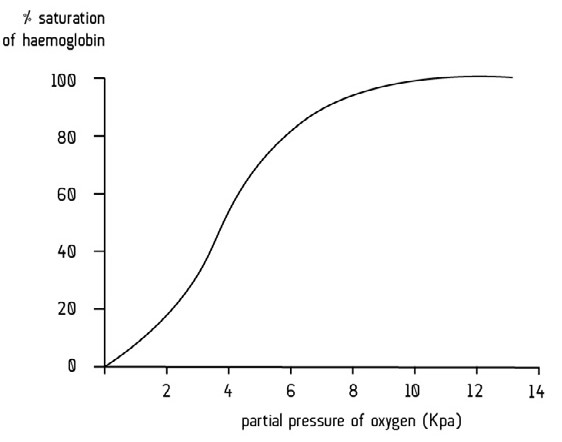
What does an oxygen dissociation curve show?
The oxygen dissociation curve shows the relationship between the oxygen partial pressure and how much oxygen is carried by haemoglobin.
What is loading / association?
When oxygen diffuses into the red blood cells and binds to the haemoglobin inside.
What is unloading / dissociation?
When haemoglobin unloads oxygen to be used in aerobic respiration at the body tissues.
The amount of loading and unloading is dependent on what?
The partial pressure of oxygen surrounding the capillaries.
What does the affinity of haemoglobin to oxygen refer to?
The ability of haemoglobin to bind to oxygen.
When does haemoglobin have a high affinity for oxygen?
When the partial pressure is high- haemoglobin binds to oxygen more readily.
When does haemoglobin have a low affinity for oxygen?
When the partial pressure is low- haemoglobin unloads oxygen more readily.
Describe the percentage saturation of haemoglobin with oxygen when the partial pressure of oxygen is high.
At high partial pressures of oxygen, haemoglobin becomes saturated with oxygen, forming oxyhaemoglobin.
Haemoglobin is said to be saturated when what?
When haemoglobin is combined with the maximum possible number of oxygen atoms.
Describe the percentage saturation of haemoglobin with oxygen when the partial pressure of oxygen is low.
At low partial pressures of oxygen, oxyhaemoglobin dissociates and unloads its oxygen for aerobic respiration.
Where is the partial pressure of oxygen high in the body?
In the lungs (alveoli).
Where is the partial pressure of oxygen low in the body?
In actively respiring tissues.
What does the partial pressure of oxygen (kPa) refer to?
The concentration of oxygen in the lungs or body tissues.
What type of curve is an oxygen dissociation curve?
S-shaped (sigmoid) curve.
What is co-operative binding?
The binding of the first oxygen molecule results in a conformational change in the structure of the haemoglobin molecule, making it easier for each successive oxygen molecule to bind; this is cooperative binding.
Explain the drawbacks of having a linear relationship between the oxygen partial concentration and % saturation of haemoglobin for oxygen.
If the relationship was linear, rather than producing an s-shaped curve, at a high PP, hemoglobin’s affinity for oxygen would be too low and oxygen would be readily released and not reach respiring tissues.
Also, at low PP, haemoglobin’s affinity would be too high and oxygen would not be released in respiring tissues.
Organisms that are adapted to live in low oxygen environments, e.g. at high altitudes, in burrows or in deep lakes, have haemoglobin that fully saturates at what ppO2?
At lower partial pressures of oxygen.
What does it mean if the dissociation curve is shifted to the left?
Haemoglobin has a higher affinity for oxygen- this means that their haemoglobin is fully saturated at lower ppO2.
Why must foetal haemoglobin have a dissociation curve to the left of the mother’s adult haemoglobin?
The foetal haemoglobin has a greater affinity for oxygen (combines more readily).
The foetal haemoglobin is more saturated with oxygen at the same partial pressure.
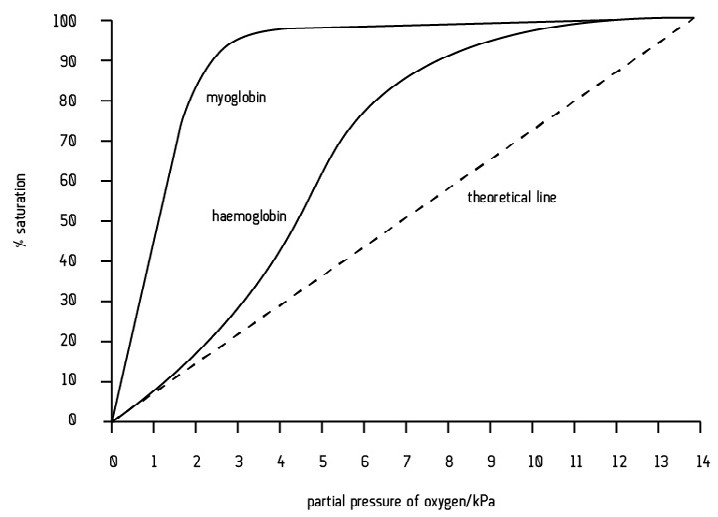
Describe the dissociation curve for another respiratory pigment, myoglobin.
Myoglobin is a muscle protein. It is far more stable than haemoglobin.
The dissociation curve is far to the left of haemoglobin.
At each partial pressure of oxygen, myoglobin has a higher percentage oxygen saturation than haemoglobin. In other words, myoglobin binds and holds onto oxygen more readily.
Myoglobin does not release oxygen as readily as haemoglobin. However, at very low oxygen partial pressures (during exercise) oxymyoglobin releases oxygen to the muscle tissues.
Myoglobin acts as an oxygen reserve in respiring muscle tissue.