A2 Carbonyls and carboxylic acids -SZ
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
What is the reaction between Acyl chloride and H20?
Carboxylic acid and Hydrochloric acid
Hydration reaction
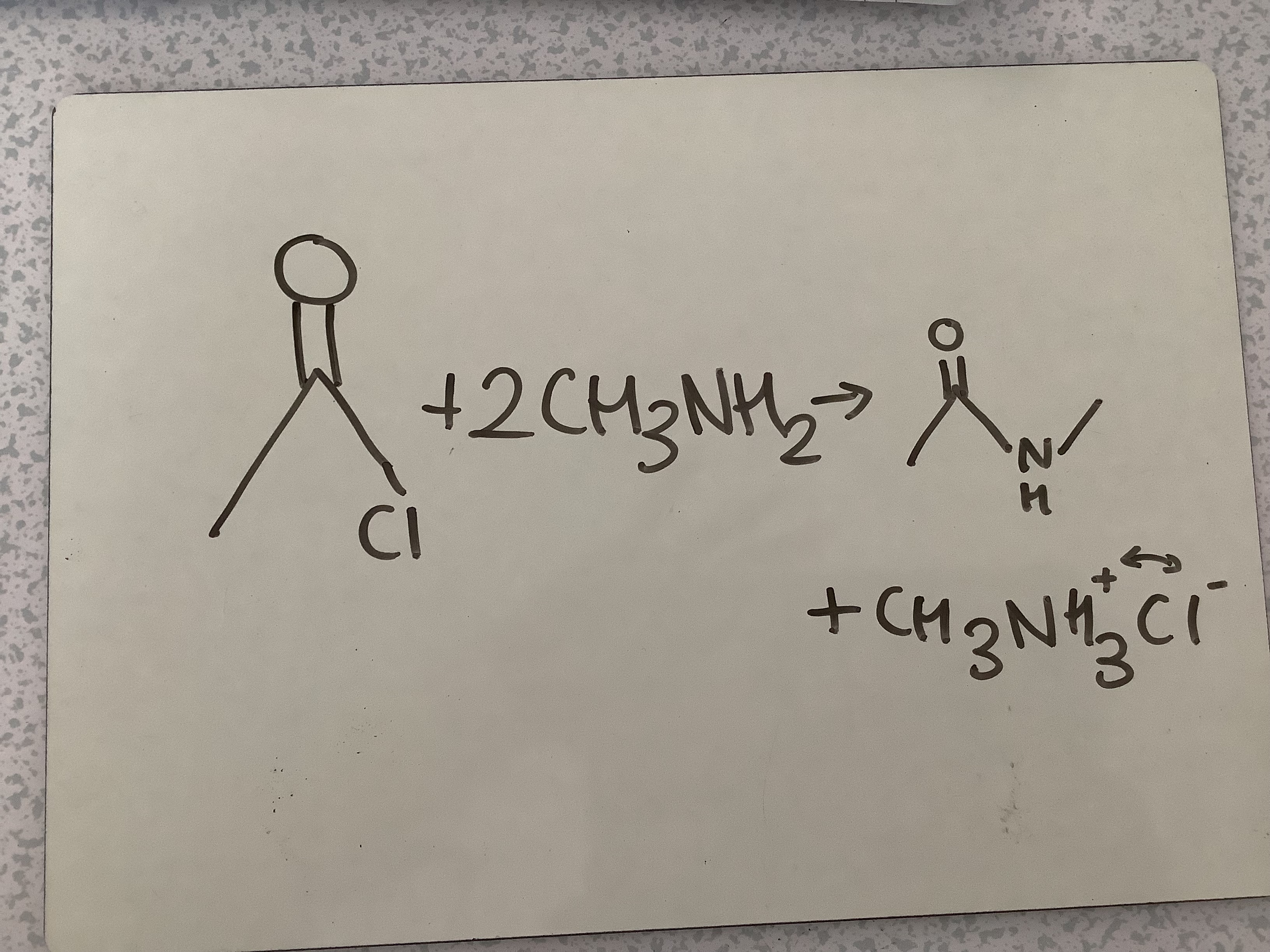
What is the reaction between Acyl chloride and 2 Amine?
Amide + Ammonium salt
Amidification
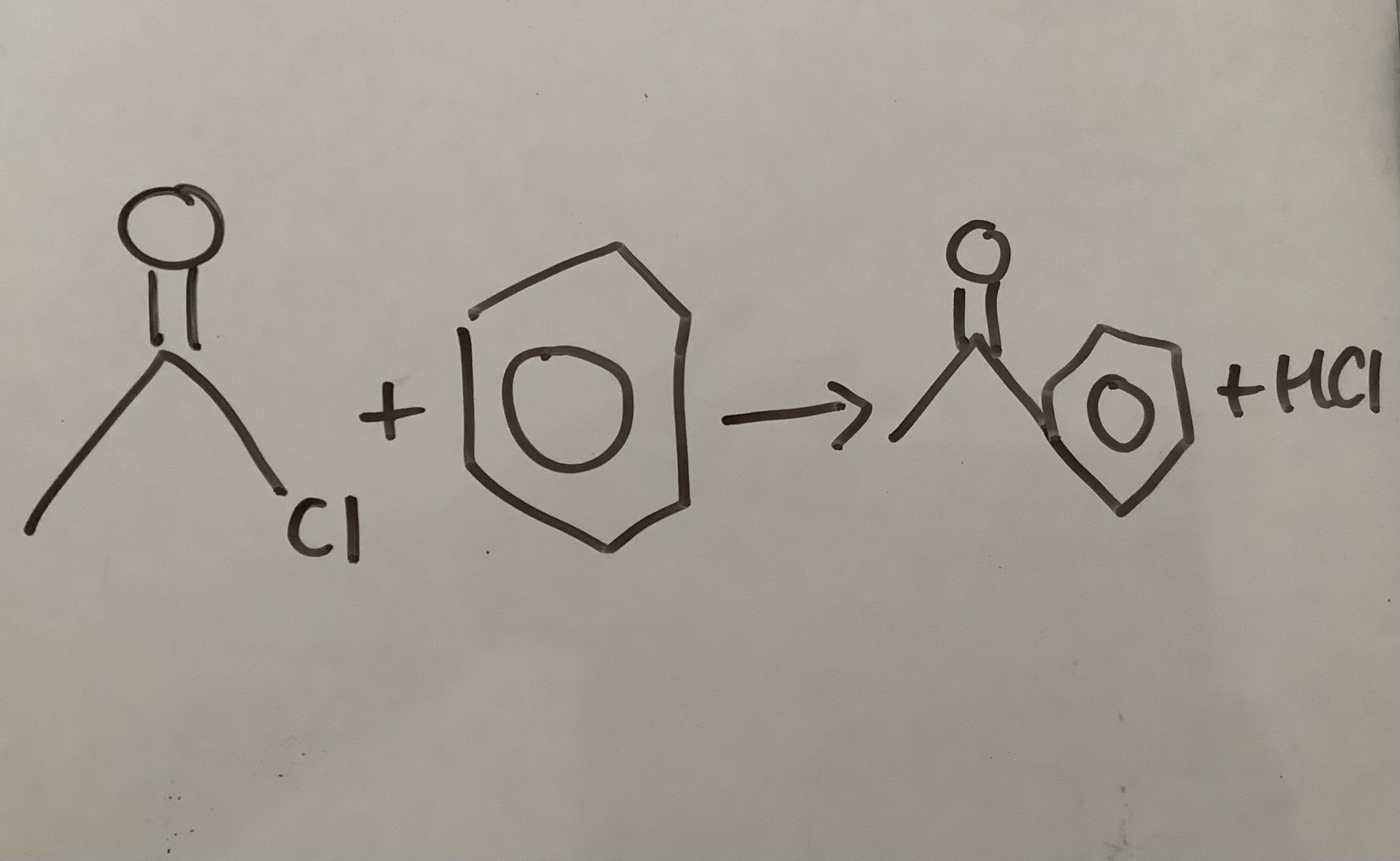
Acyl chloride + Benzene —>
Conditions?
Halogen carrier (AlCl3) as benzene a localised area of electron density which isn’t on its own reactive enough to react with Acyl Chloride
Ketone + Benzoate
Acylation
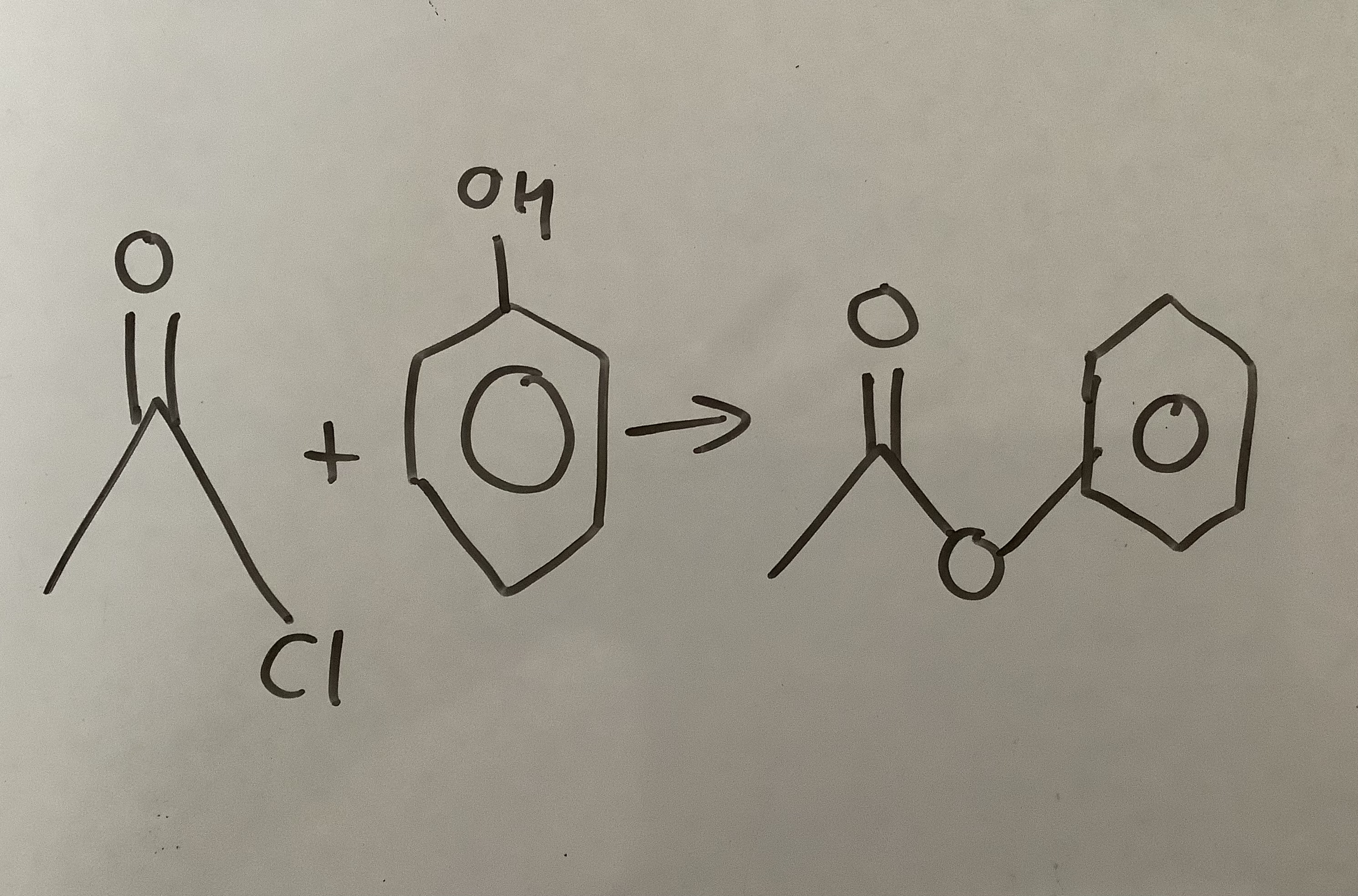
Acyl chloride + Alcohol —→
Ester + Hcl
Esterification
Test for Aldehydes and Ketones: + or -?
K2Cr2O7 acidified
Positive (Aldehyde): The color changes from orange to green, as the aldehyde is oxidized to a carboxylic acid
Negative (Tertiary Alcohol): The solution remains orange.
2,4 DNP test: + or -?
Yellow, orange or red precipitate —> if an aldehyde or ketone present (as shows presence of the CO bond)
Where is the carbonyl group in the chain for aldehyde’s?
It is at the end of the carbon chain.
Where is the carbonyl group in the chain for ketone’s?
It is within the chain.
What type of addition do carbonyls undergo?
Nucleophillic addition
Functional group of aldehyde?
RCHO
Functional group of ketone
RCOR
What IMF’s do molecules with CO group have?
Permanent dipole-dipole due to the polar C=O bond (O is the delta minus)
CO compounds solubility in H2O?
They are soluble in water
H bonds between H2O molecules and Oxygen of C=O
As the C chain length increases , the solubility decreases
Which bond in C=O compounds is usually involved in reactions and why?
C=O
This is due to the polarity of the bond (the difference in electronegativity between C and O)
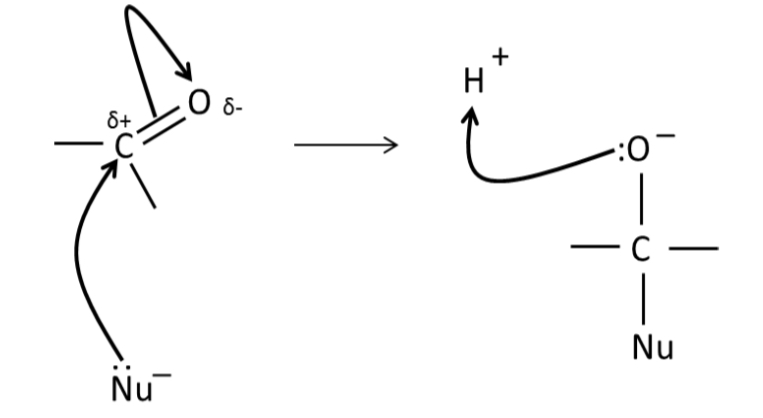
Mechanism for nucleophillic addition of CO compound?
Mechanism for nucleophillic addition of HCN compound?
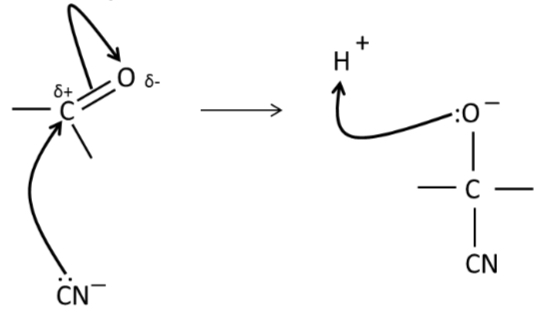
What is the name of the product when HCN is added to a CO compound?
Hydroxynitriles (have OH and CN groups)
What is in Tollens solution?
Silver ions in dilute ammonia
What happens when an aldehyde is added to Tollen’s reagent?
Silver mirror forms
Ag+ is reduced to Ag (s)
Amine + Carboxylic acid
Amide + H20
Carboxylic acid +SOCl2
Acyl chloride + HCl + SO2
Alc + Carb acid
Ester and H2O
Z Carb acid
+ acid anhydride