4.4.1.3 Extraction of metals and reduction
0.0(0)
Card Sorting
1/19
Last updated 9:16 AM on 12/13/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
1
New cards
ore
a rock containing enough metal to make it economic to extract the metal
Rocks rich in useful metals are called ores- gold ore, metal ore
Rocks rich in useful metals are called ores- gold ore, metal ore
2
New cards
native
unreactive metals, found as elements in nature
3
New cards
Electrolysis
Electrolysis is needed to extract these metals because their oxides cannot be reduced by carbon
4
New cards
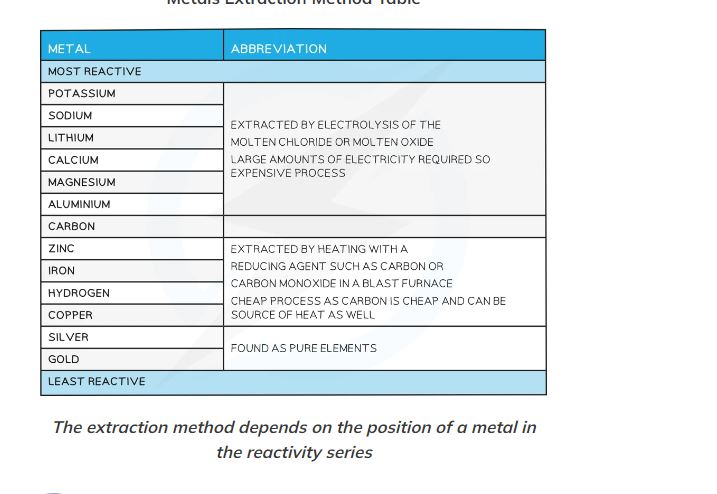
ways of extraction from elements in the reactivity series
a more reactive element will push out (displace a less reactive element in its compound
5
New cards
Where are most metals found?
Earth’s Crust
6
New cards
What is panning?
physical separation of
native metals
native metals
7
New cards
Give three factors that make it economically viable to extract metal?
How easy it is to extract from its ore
How much metal the ore contains
The changing demands for a particular metal.
How much metal the ore contains
The changing demands for a particular metal.
8
New cards
How are most metals extracted the earth?
Mining
9
New cards
Why is hydrogen not generally used to extract metals?
More expensive than carbon
10
New cards
Why is carbon used to extract metals?
Cheap and readily available
11
New cards
How do we extract metals above carbon in the reactivity series and why can’t we use carbon to extract them?
Electrolysis
Carbon is below them in the reactivity series (less reactive) so cannot displace them.
Carbon is below them in the reactivity series (less reactive) so cannot displace them.
12
New cards
Explain how reduction by carbon works?
During the reaction, the metal in the ore is displaced from
its non-metal anion because carbon is more reactive than
the metal.
13
New cards
Why don’t we generally use “reduction” to extract metals above carbon in the reactivity series and what is the only exception?
more expensive than electrolysis and is only
used on a commercial scale for the extraction of titanium.
used on a commercial scale for the extraction of titanium.
14
New cards
Give two alternative names for carbon
) Coke and Charcoal
15
New cards
What is the equation for the reduction of tungsten? Why is carbon not used?
Carbon reacts with tungsten to form tungsten carbide.

16
New cards


17
New cards
metal oxide + carbon
metal oxide + carbon → metal + carbon dioxide
18
New cards
iron(III) oxide + carbon → iron + carbon dioxide
2Fe2O3(s) + 3C(s) → 4Fe(l) + 3CO2(g)
19
New cards
how is iron extracted
a large reaction vessel called a blat furnace
20
New cards
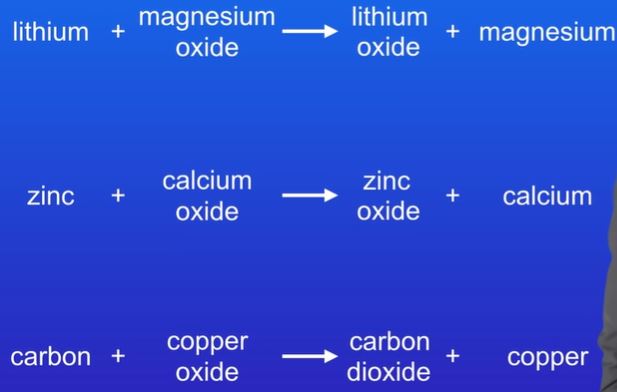
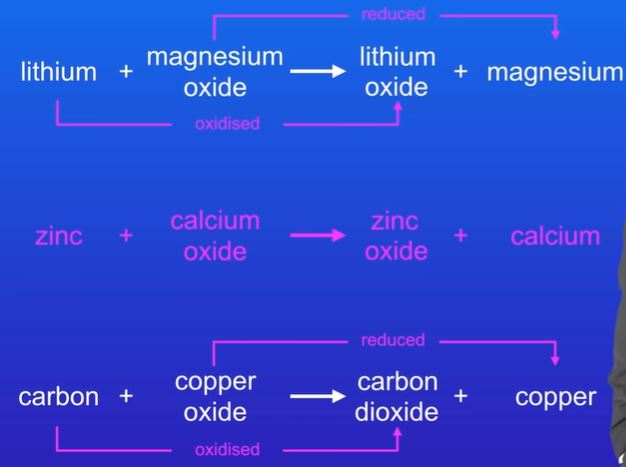
explain which chemicals are being oxidised and which are being reduced and which is not possible