Atmospheric chemistry
0.0(0)
Card Sorting
1/161
Earn XP
Description and Tags
Last updated 6:06 AM on 11/12/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
162 Terms
1
New cards
Regions of the Atmosphere
troposphere, stratosphere, traupopause
2
New cards
Troposphere
- the lowest atmospheric layer; from 4 to 11 miles high
- the temperature of this layer decreases as altitude increases
- Most of the atmospheres water is here
- the temperature of this layer decreases as altitude increases
- Most of the atmospheres water is here
3
New cards
stratosphere
- the atmospheric layer between the troposphere and the mesosphere
- Most of the ozone is in the stratosphere and the most is 20-30km above the surface of the earth.
- IR Absorption occurs here and as a result in is very warm that increases as altitude increases
- because tropopause is very cold and troposphere decreases in temperature as altitude increases the increase in temperature in stratosphere is a temperature inversion
- very little water
- Most of the ozone is in the stratosphere and the most is 20-30km above the surface of the earth.
- IR Absorption occurs here and as a result in is very warm that increases as altitude increases
- because tropopause is very cold and troposphere decreases in temperature as altitude increases the increase in temperature in stratosphere is a temperature inversion
- very little water
4
New cards
tropopause
- boundary between troposphere and stratosphere
- Cold (~ -60 degrees celcius)
- Cold (~ -60 degrees celcius)
5
New cards
Light
Can be considered a wave but also have particle-like properties in that it is absorbed (or emitted) by matter only in finite packets now called photons.
6
New cards
Einstein-Planck Equation
E=hv=hc/wavelength
7
New cards
How does energy transfer change with wavelength?
The shorter the wavelength of light the greater the energy it may transfer to matter when absorbed.
8
New cards
How to X-Rays affect molecules?
causes ionization (ejection of core electrons) to occur
9
New cards
How do UV Rays affect molecules?
Bonds break and molecules dissociate
10
New cards
How does Visible light affect molecules?
Cause absoprtion/reflection of light/photons (you'll see colours)
11
New cards
How does Infrared affect molecules?
Molecules will vibrate causing release of heat.
12
New cards
How do microwaves affect molecules?
molecules will rotate
13
New cards
Absorption
When light absorbed in the volume of a material. It doesn't pass through the object.
14
New cards
Pressure in Atmosphere vs. Altitude (Draw)
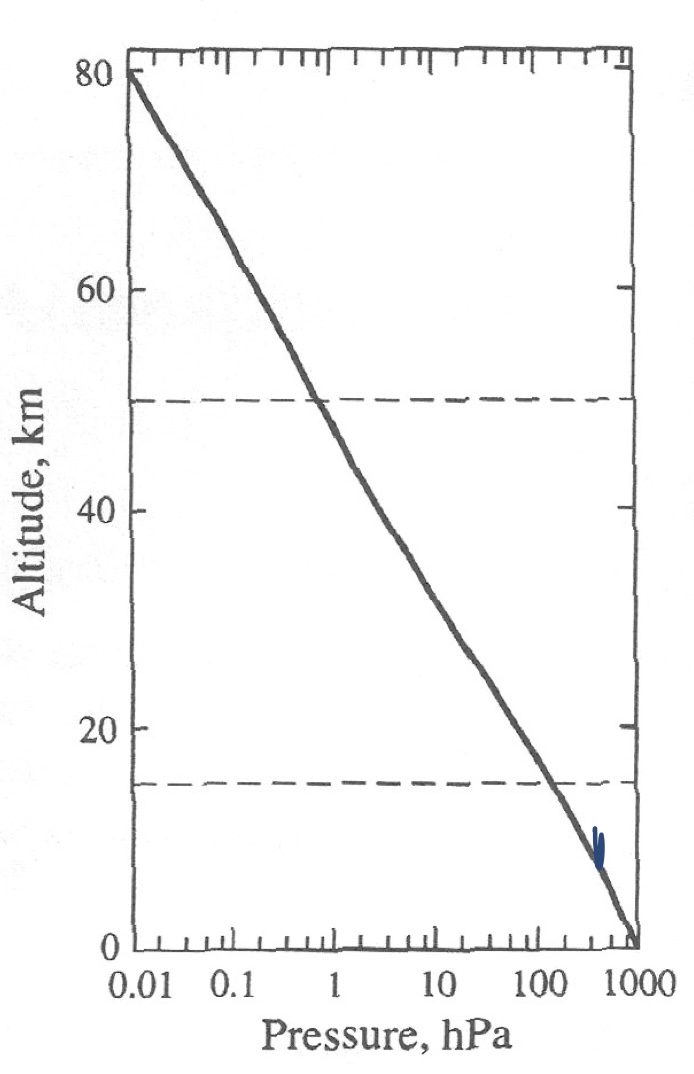
15
New cards
Temperature in Atmosphere vs. Altitude (draw)
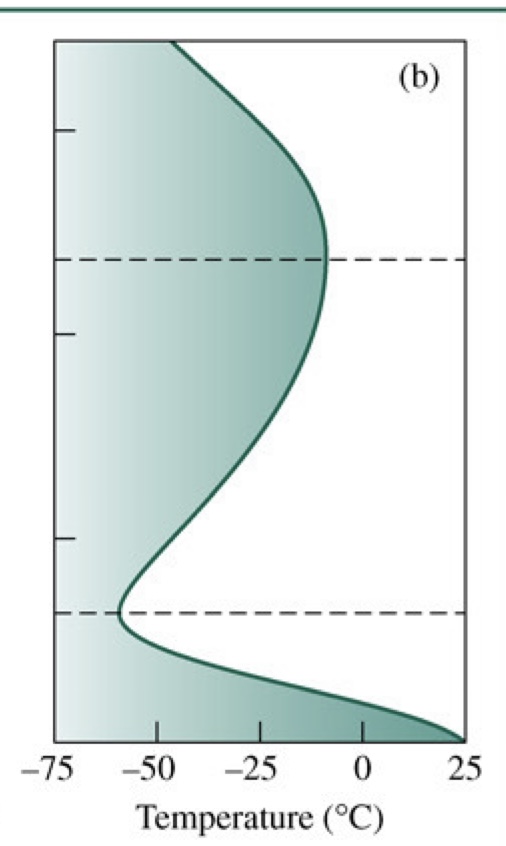
16
New cards
Ozone in atmosphere vs. Altitude (draw)
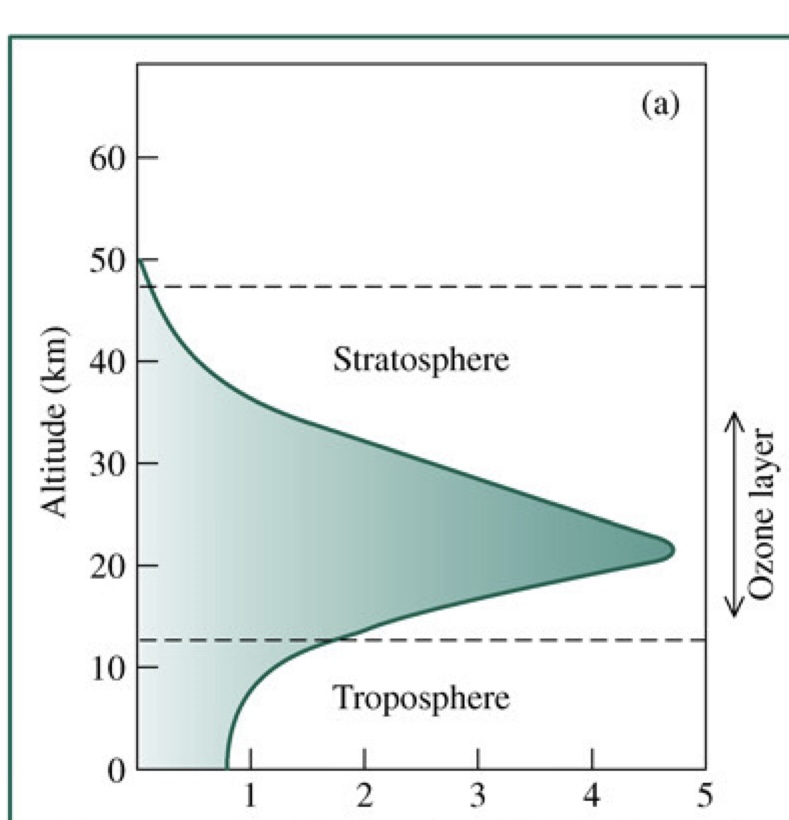
17
New cards
Mole fraction of water in atmosphere vs. Year (draw)
too variable because of positive and negative feedback it would be a crazy graph.
18
New cards
Mole fraction of CO2 in atmosphere vs. Year (draw)
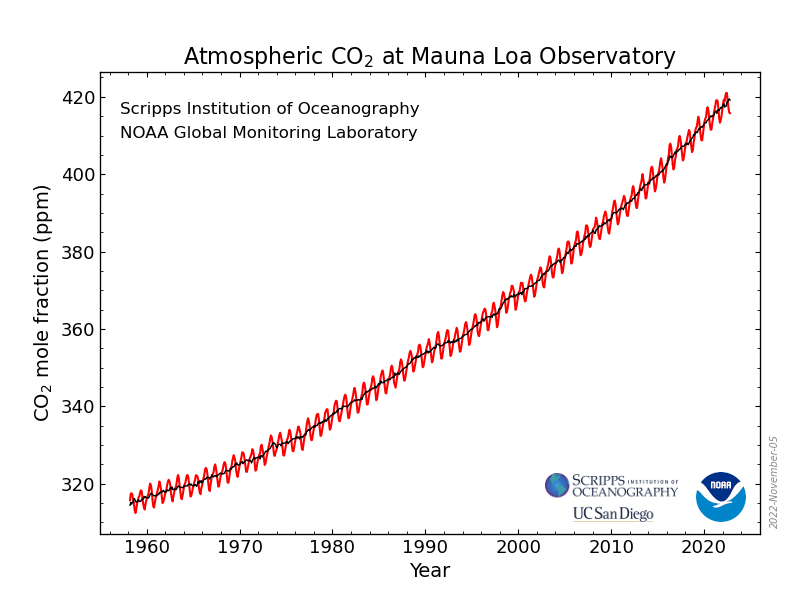
19
New cards
Mole fraction of CH4 in atmosphere vs. Year (draw)
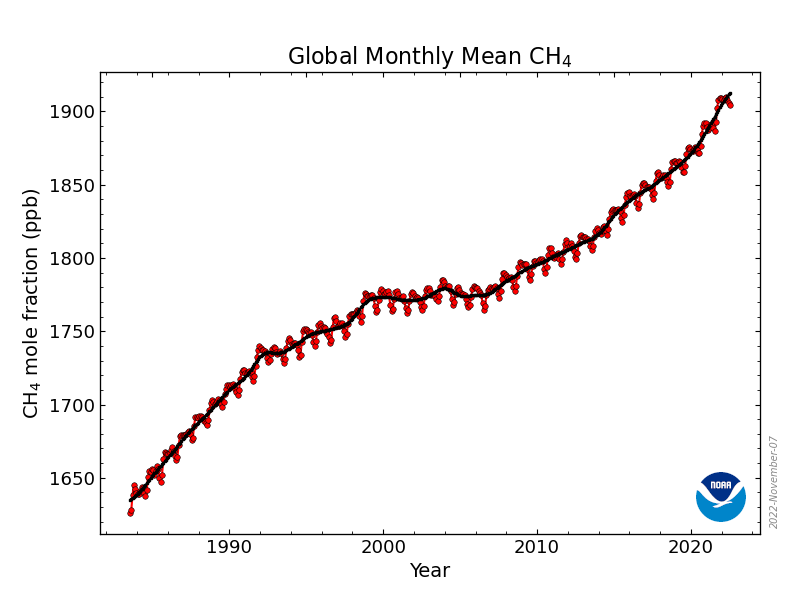
20
New cards
Mole fraction of N2O in atmosphere vs. Year (draw)
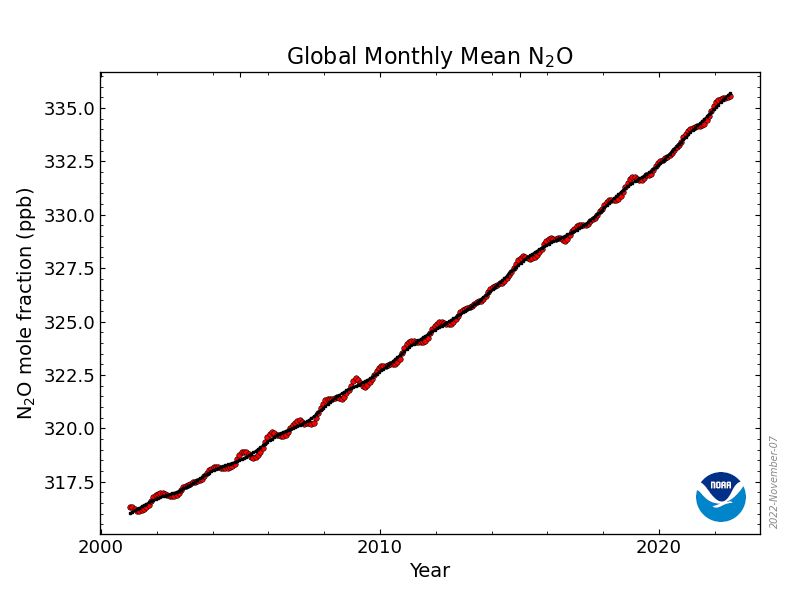
21
New cards
Mole fraction of SF6 in atmosphere vs. Year (draw)
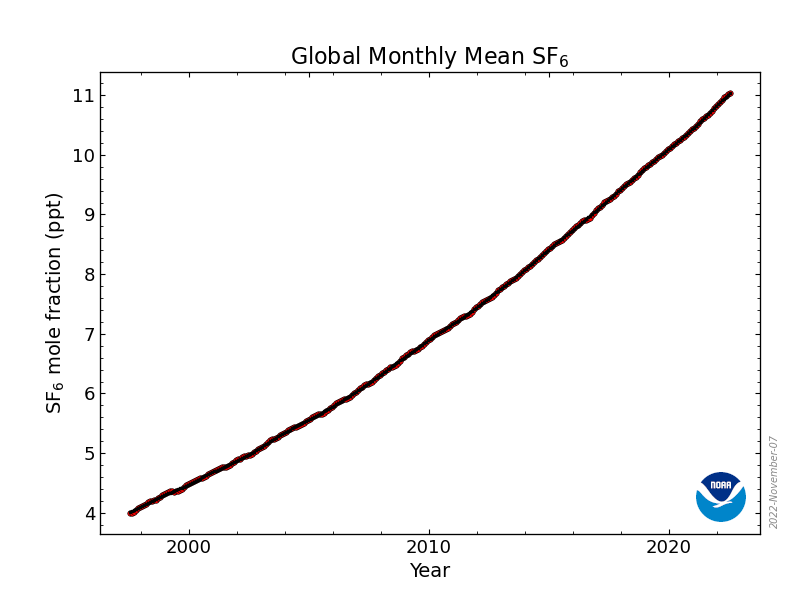
22
New cards
Transmitted light
Light emerges propagating in the same direction as the incident light. It does pass through the object.
23
New cards
Scattered light
Emerges in a different direction from the incident light.
24
New cards
Beer-Lambert Law equation
I/I0=e^-sigma*xnxL
where, I= intensity transmitted
I0=intensity of incident light.
Sigma=absorption cross section
N=number density of absorbing molecule
L=optical path length
e=euler's number
where, I= intensity transmitted
I0=intensity of incident light.
Sigma=absorption cross section
N=number density of absorbing molecule
L=optical path length
e=euler's number
25
New cards
Rayleigh scattering
- Scattering of radiation by gases, at low wavelengths light is scattered more efficiently than at high wavelengths
-O2 and O3 are big contributors to this
-O2 and O3 are big contributors to this
26
New cards
Solar radiation
Light from the sun from the atmosphere is mostly infrared then visible then ultraviolet.. Most of it is absorbed by the atmosphere but some transmits to earth and some gets scattered to earth at space.
27
New cards
Conditions for dissociation
1- enough energy to dissociate
2- molecules need to actually absorb the energy
2- molecules need to actually absorb the energy
28
New cards
Absorption
Atoms and molecules absorb light and electrons become excited but this is unstable and they will return to ground state. Or the molecule can undergo a chemical reaction that releases a photon or heat,
29
New cards
bond dissociation energy
the energy required to break the bond between two covalently bonded atoms. Like O2→ 20
30
New cards
Impact of excess UV radiation
DNA mutations, shin cancer (melanoma), and death.
31
New cards
Ratio for ozone to uv-b intensity on the ground
1% decrease in ozone results in 2% increase of UV-B
32
New cards
DNA mutations
DNA can absorb UV-B light
33
New cards
Skin cancer
Over exposure to UV-B light can lead to skin cancer
34
New cards
Death
Sun stroke, shin cancer, sun burns can all lead to death
35
New cards
Sunscreen
- Full spectrum sunscreen protects against UV-A and UV-B light.
- Sunscreens often contain organic substances that absorb or scatter light.
- zinc oxide and titanium dioxide are nano particles also included that appear transparent due to their size.
- these ingredients may produce byproducts like OH radicals
- SPF 15 means 15 minutes of protection against UV-B
- A new star rating is being introduced to define effectiveness against UVA.
- Sunscreens often contain organic substances that absorb or scatter light.
- zinc oxide and titanium dioxide are nano particles also included that appear transparent due to their size.
- these ingredients may produce byproducts like OH radicals
- SPF 15 means 15 minutes of protection against UV-B
- A new star rating is being introduced to define effectiveness against UVA.
36
New cards
Cataracts.
When the eye is exposed to uv light the cornea and lens will filter 99% of uv light. Absorption of uv can produce reactive molecules that attach to structural molecules in the eye and produce cataracts
37
New cards
Crop failure
Uv-b exposure can effect photosynthesis.
38
New cards
Reaction mechanism
A sequence of elementary reactions by which overall chemical change occurs (and allows to deduce the rate law)
39
New cards
Catalyst
A molecule that lowers the reaction energy barrier (and speeds up the reaction) without being consumed or created in the net reaction.
40
New cards
Catalytic cycle
A reaction sequence in which a chemical family, catalyst, catalizes a net reaction.
41
New cards
Chapman Mechanism
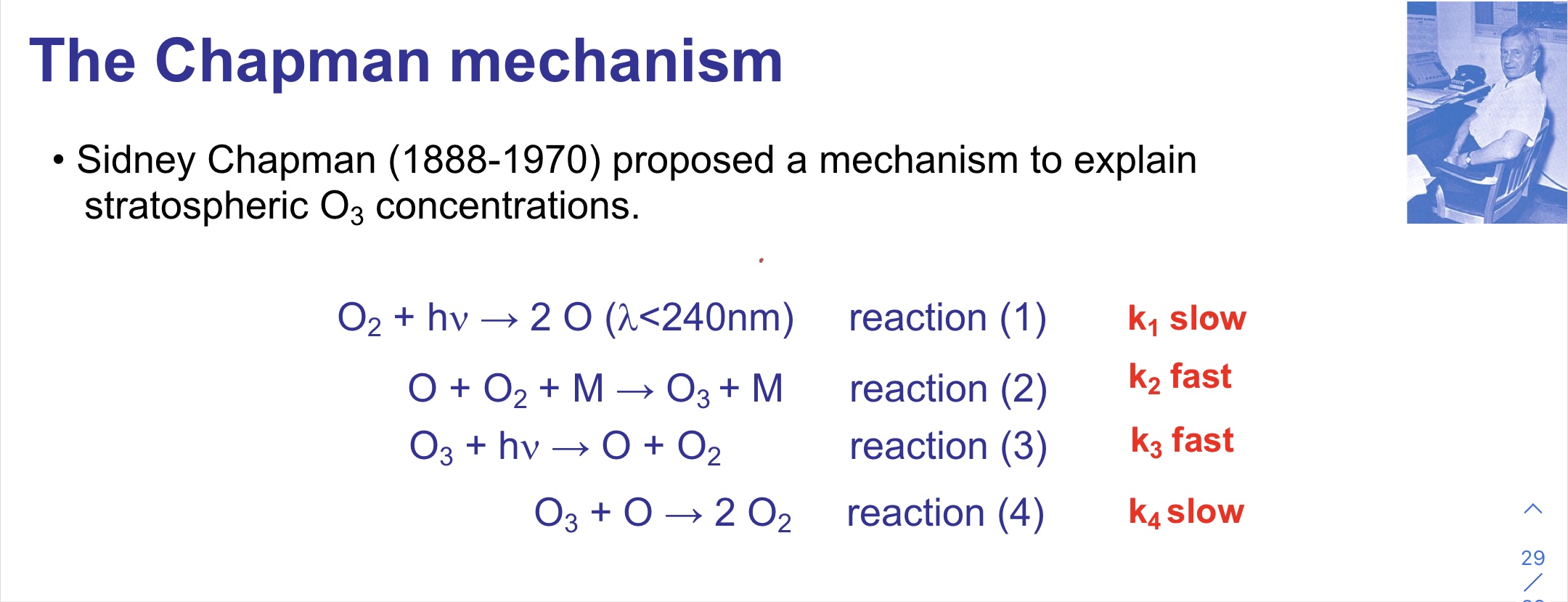
42
New cards
What does the Chapman mechanism do?
Qualitatively predicts altitude dependence of o3 but does not account for catalytic destruction of the stratospheric ozone
43
New cards
Catalyst in catalytic destruction of ozone
1. OH hydroxyl group
2.NO nitric oxide radical
3. Reactive halogen radicals chlorine and bromine
2.NO nitric oxide radical
3. Reactive halogen radicals chlorine and bromine
44
New cards
OH catalytic destruction of the ozone
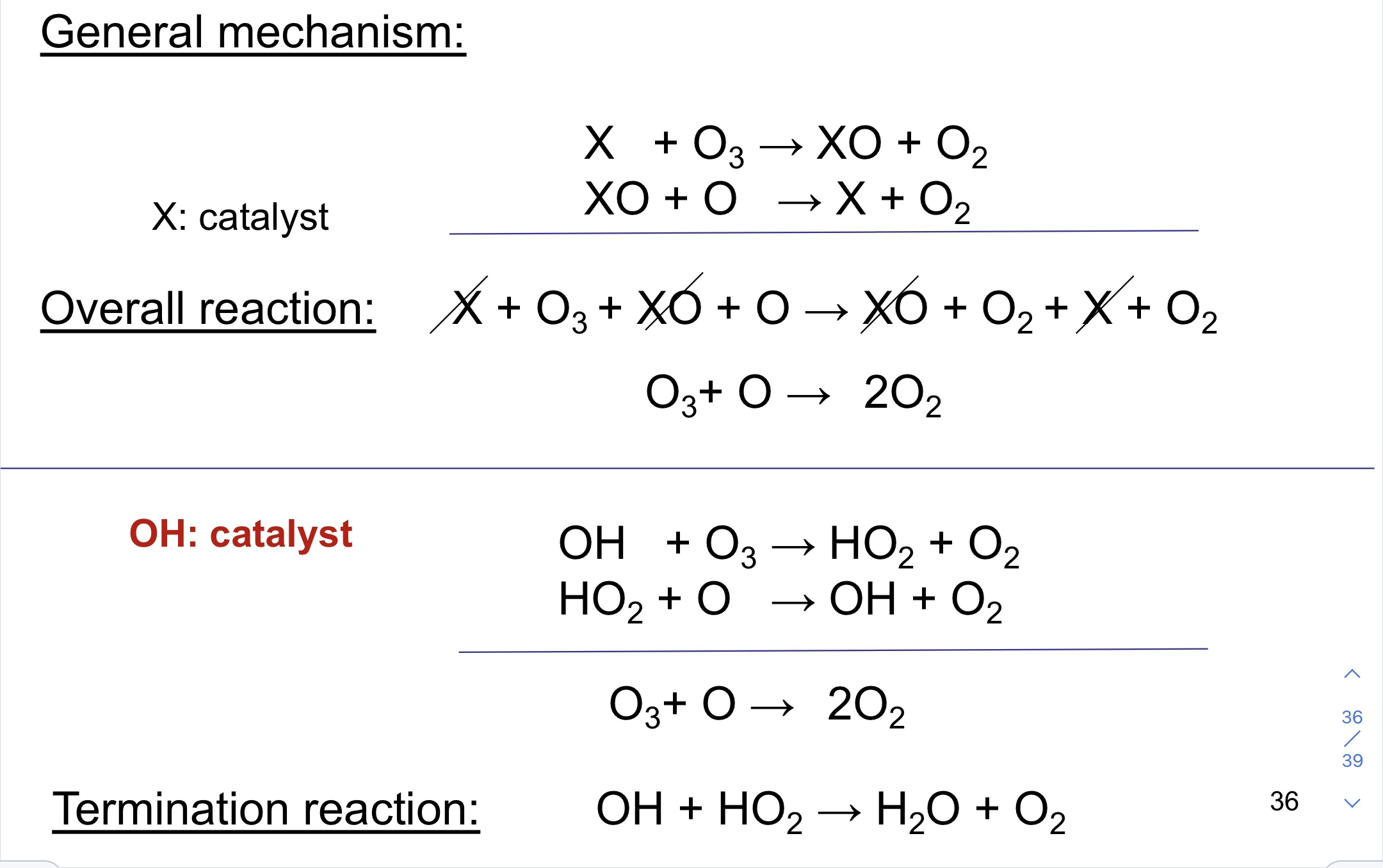
45
New cards
Nitric oxide radicals catalytic destruction of the ozone
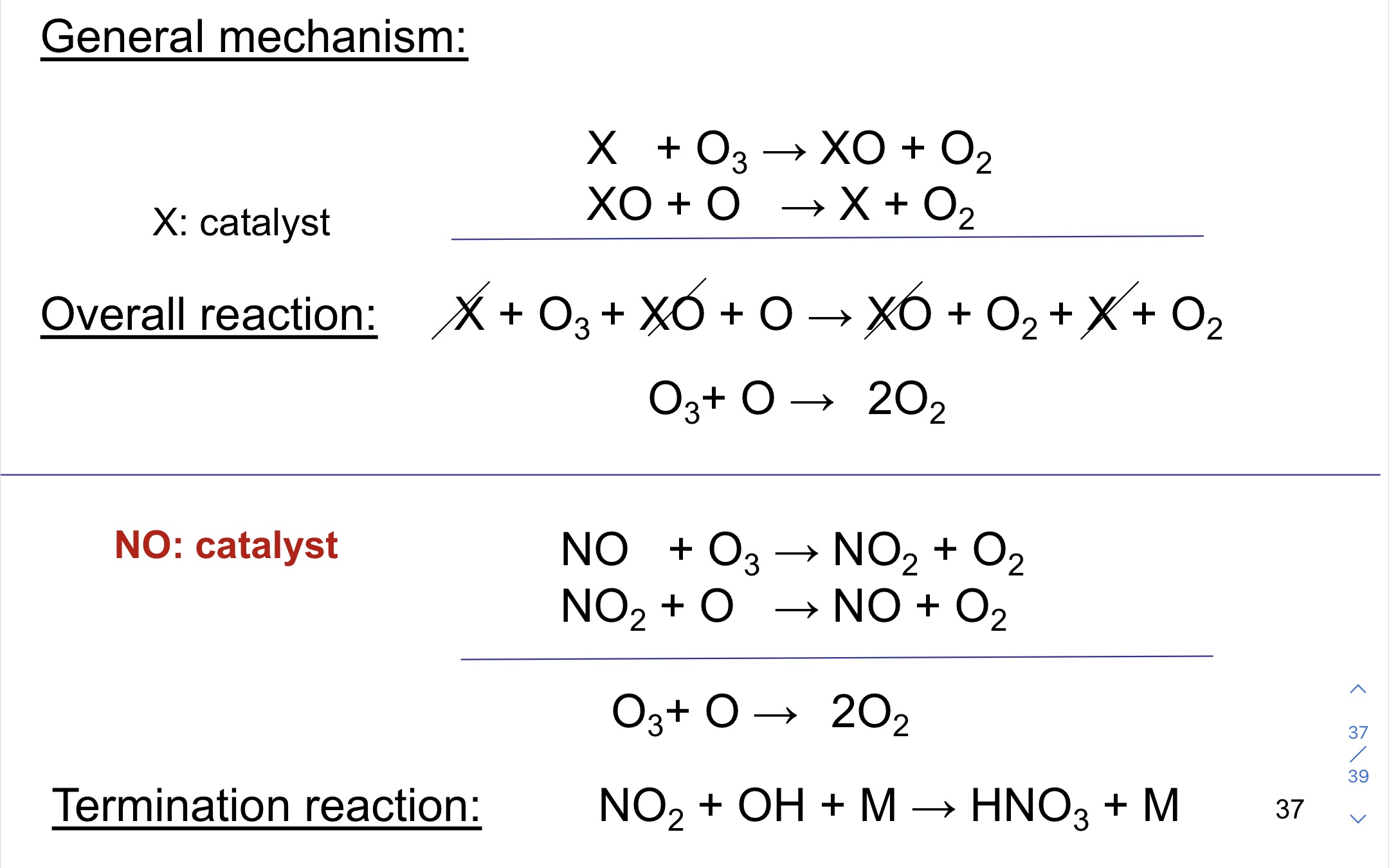
46
New cards
Halogen Radicals
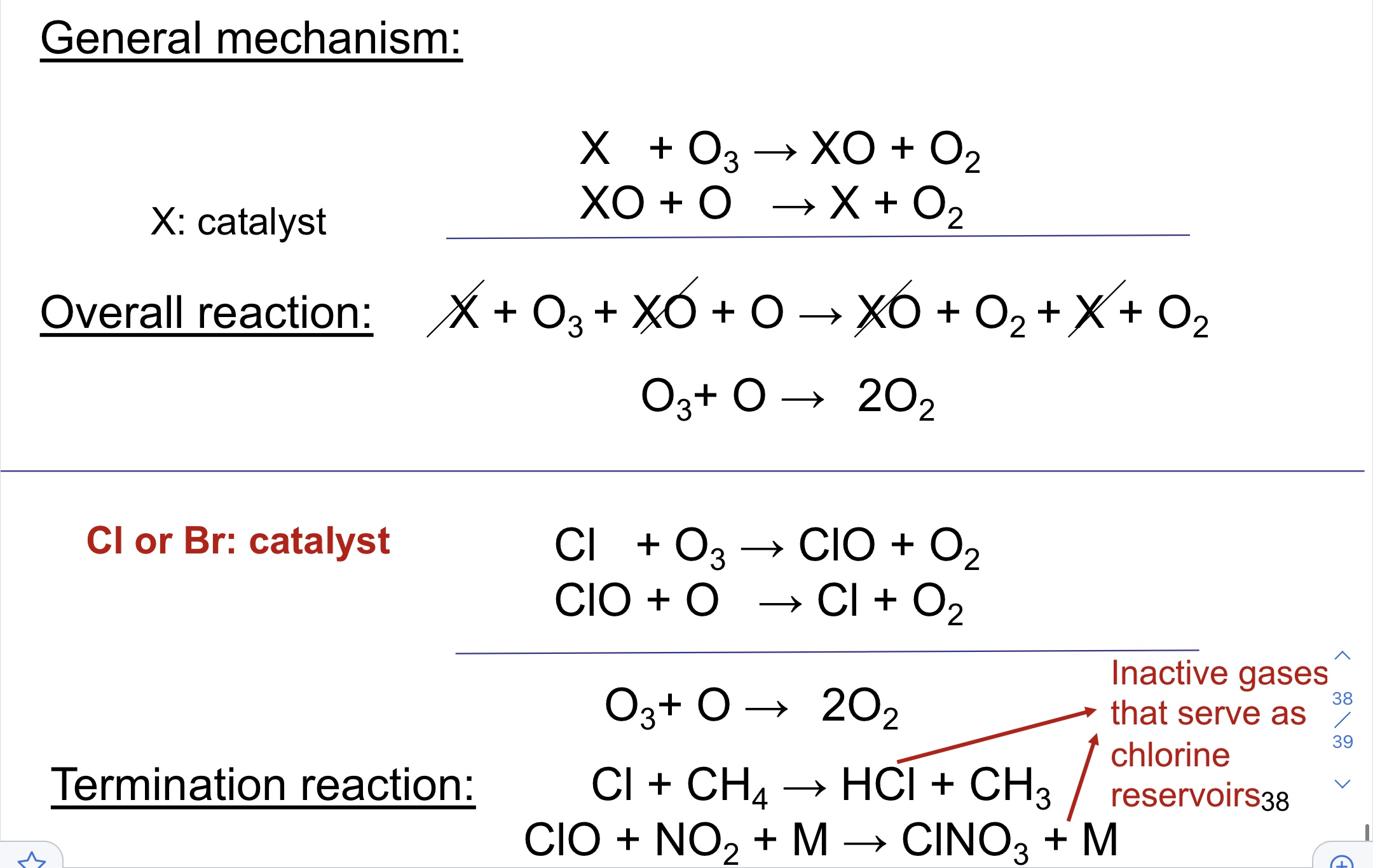
47
New cards
General mechanism for destruction of Stratospheric ozone.
X: catalyst
X + O3 -> XO + O2
XO + O -> X + O2
Overall: O3 + O -> 2O2
X + O3 -> XO + O2
XO + O -> X + O2
Overall: O3 + O -> 2O2
48
New cards
Steady state
Rate of formation = rate of destruction
d[O]/dt = 0 = P-L or 2k_1[O_2] + k_3[O_3] = k_2[O_2][M][O] + k_4[O_3][O]
d[O]/dt = 0 = P-L or 2k_1[O_2] + k_3[O_3] = k_2[O_2][M][O] + k_4[O_3][O]
49
New cards
Inactive gases
HCl, ClNO3
50
New cards
Reservoir species
Catalytically inactive molecules containing chlorine available in the stratosphere. This accounts for most of the chlorine in the stratosphere. They are relatively long-lived and they tie up large amounts of active, short lived species but can slowly rerelease them when activated.
51
New cards
Chlorofluorocarbons (CFCs)
- A compound containing only C, F, and Cl
- Nontoxic
- Non flammable
- volatile
- nonbiocumulative
- inert
- widely used since the 1940s in fridges and spray cans
- Nontoxic
- Non flammable
- volatile
- nonbiocumulative
- inert
- widely used since the 1940s in fridges and spray cans
52
New cards
Chlorofluorocarbon story
- Back in the day, 1974, the solution to pollution was dilution so CFCs were spread around the were
- the lack of tropospheric sinks with a long lifetime which meant that they eventually mixed with the stratosphere.
- in the stratosphere there is a lot more uv light and CFCs would photo- dissociate
- in 1985 large losses of ozone in Antarctica
- in mid 1990s, holes were extending out to Australia and New Zealand
- the holes required sunlight to form
- the effect was seen at lower latitudes but they were less pronounced.
- the lack of tropospheric sinks with a long lifetime which meant that they eventually mixed with the stratosphere.
- in the stratosphere there is a lot more uv light and CFCs would photo- dissociate
- in 1985 large losses of ozone in Antarctica
- in mid 1990s, holes were extending out to Australia and New Zealand
- the holes required sunlight to form
- the effect was seen at lower latitudes but they were less pronounced.
53
New cards
Dobson unit
Quantitative measure of ozone column abundance. The height of the layer of pure gaseous ozone in units of 10-5m that one would have, if one took all the O3 and compressed it to a column at 1 atm and 273K
54
New cards
How do ozone holes form
-they form at South Pole @ very cold temperatures
- pressure drop causes a polar vortex that isolates the south pole
- it is so cold that polar stratospheric clouds (PSCs) form made up of ice crystals with sulfuric and nitric acid the crystals serve as important catalytic surfaces.
- reservoir species like ClNO3 and HCl will react with PSC's surface to produce active chlorine that destroys the ozone
- catalytic destruction requires sunlight so this occurs in spring
-Only when the polar vortex ends and temperatures rise do the active chlorines convert back to inactive chlorine,
- pressure drop causes a polar vortex that isolates the south pole
- it is so cold that polar stratospheric clouds (PSCs) form made up of ice crystals with sulfuric and nitric acid the crystals serve as important catalytic surfaces.
- reservoir species like ClNO3 and HCl will react with PSC's surface to produce active chlorine that destroys the ozone
- catalytic destruction requires sunlight so this occurs in spring
-Only when the polar vortex ends and temperatures rise do the active chlorines convert back to inactive chlorine,
55
New cards
How to fix the ozone holes.
1- give up on refrigeration
2 - replace CFCs
2 - replace CFCs
56
New cards
Replacing CFCs
- If we stick with Cl and Br containing compounds we want ones with a shorter tropospheric lifetime than CFCs, less volatile but are still good refrigerants.
- add a functional group to the molecule that is prone to reaction
- main tropospheric sink for most compounds is reaction with hydroxyl radical so big driving force.
-Which functional group to add? Hydrogen to make hydrochlorofluorocarbon (HCFCs)
- add a functional group to the molecule that is prone to reaction
- main tropospheric sink for most compounds is reaction with hydroxyl radical so big driving force.
-Which functional group to add? Hydrogen to make hydrochlorofluorocarbon (HCFCs)
57
New cards
hydrochlorofluorocarbon (HCFCs)
- Compound containing H and only C, F, and Cl as other elements
- nontoxic
- nonflammable
-Volatile
- non bioaccumulative
- not as inert as CFCs, but still good refrigerants
- also known as class Ii ozone depleting substance.
- nontoxic
- nonflammable
-Volatile
- non bioaccumulative
- not as inert as CFCs, but still good refrigerants
- also known as class Ii ozone depleting substance.
58
New cards
Problem with HCFCs
Not quite short lived enough it would be better to avoid chlorine and bromine alto their
59
New cards
Hydrofluorocarbons (HFCs)
Tropospheric short lifetime
60
New cards
Problem with HFCs
- Their degradation products HF, and CF3COOH are toxic
61
New cards
International treaties:
-Montreal protocol (1987)
-purpose to ban CFCs
- gradual ban of HCFCS
- some countries initially exempted India and China
- halons contain Br, used in fire extinguishers, were also banned and phased out
- called for ozone assessments
- predicts ozone depletion to return to 1960 levels
-purpose to ban CFCs
- gradual ban of HCFCS
- some countries initially exempted India and China
- halons contain Br, used in fire extinguishers, were also banned and phased out
- called for ozone assessments
- predicts ozone depletion to return to 1960 levels
62
New cards
Southern Hemisphere 2020
One of the largest and deepest holes since ozone monitoring occurred. The hole formation was driven by a strong, stable, and cold polar vortex.
63
New cards
Recent northern hemisphere.
First whole appeared in 2011 and Canada decided to reduce ozone monitoring we were the country with longest record of atmospheric ozone levels that is threatened by this new hole.
64
New cards
What reaction from Chapman does not happen in the stratosphere?
O2 -> 2O (wavelength
65
New cards
Can 03 be produced in the troposphere?
No there is not enough uv radiation in the troposphere to generate O3 with Chapman. Instead there is a low amount of transport of stratospheric 02 to troposphere. There are efficient sinks for 03 and therefore another way to produce it
66
New cards
Production of ozone in the stratosphere.
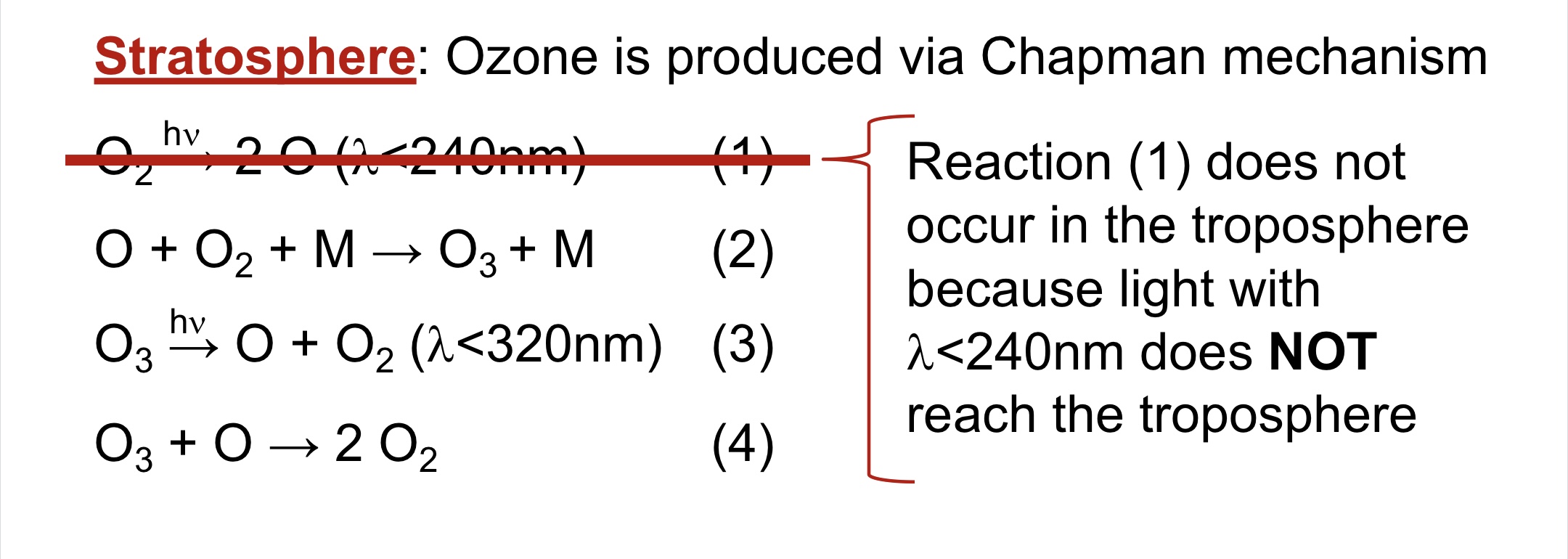
67
New cards
Production of ozone in the troposphere
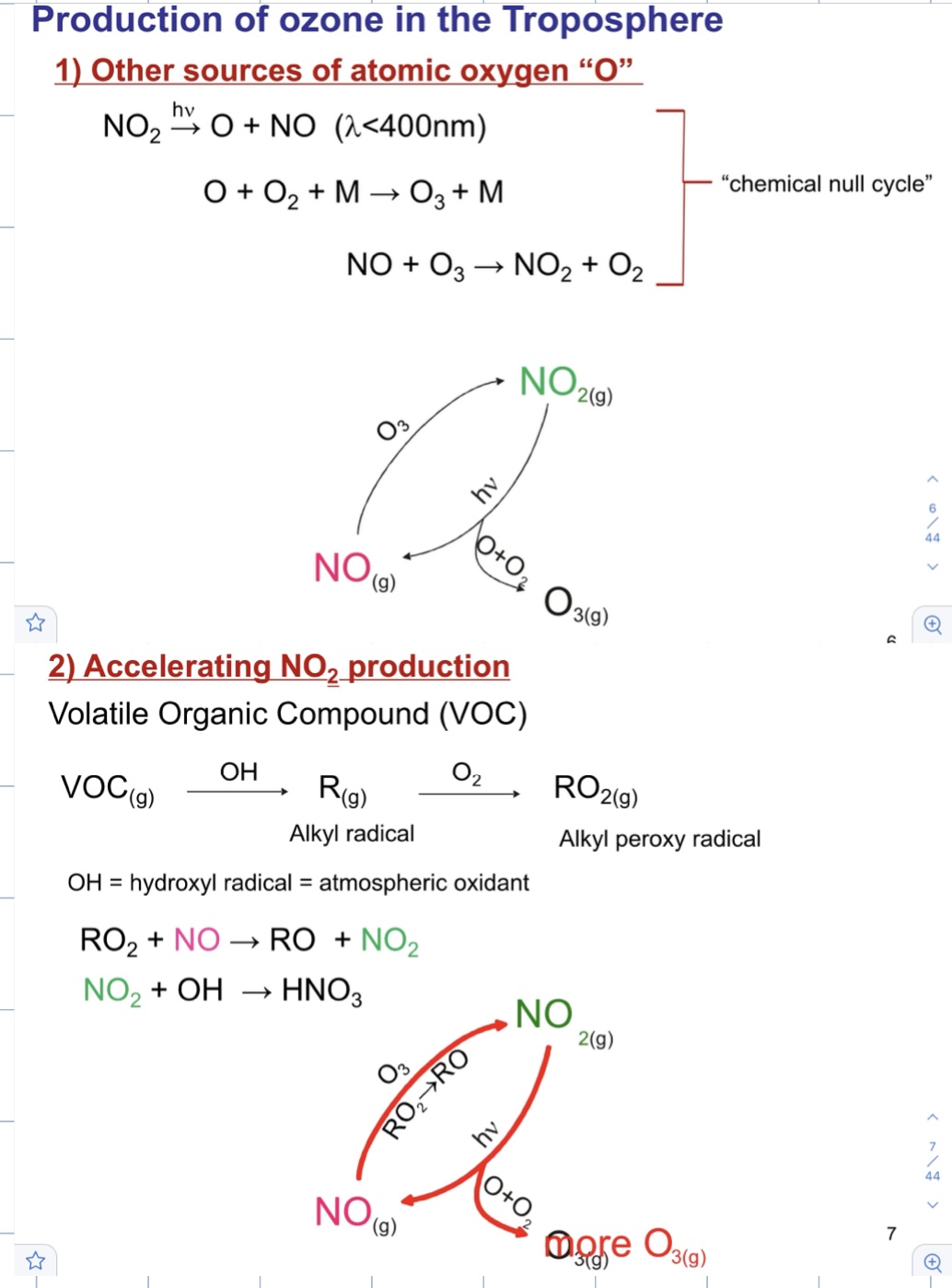
68
New cards
Many roles of ozone
- Source of the hydroxyl radical (OH), the primary atmospheric oxidant (OH is the detergent of the atmosphere)
-oxidizes unsaturated VOCS (alkenes and alkines)
- is a greenhouse gas (contributes to warming of the surface)
- is toxic and an important pollutant in urban air
-easily quantified indicator of secondary air pollution. (stratospheric ozone = good, tropospheric ozone = bad)
-oxidizes unsaturated VOCS (alkenes and alkines)
- is a greenhouse gas (contributes to warming of the surface)
- is toxic and an important pollutant in urban air
-easily quantified indicator of secondary air pollution. (stratospheric ozone = good, tropospheric ozone = bad)
69
New cards
Good vs. Bad ozone
stratospheric ozone = good, tropospheric ozone = bad
70
New cards
VOC
- Volatile organic Compound
- most have low molecular weight
- which means they have high vapour pressure
- most have low molecular weight
- which means they have high vapour pressure
71
New cards
Sources of Natural VOC
Plants and trees
72
New cards
Anthropogenic
Man made
73
New cards
Sources of Anthropogenic VOC
gasoline and industry
74
New cards
Sources of Natural NOx
Lightning, bacteria in soil biomass, and biomass burning.
75
New cards
Secondary pollutants: aldehydes and peroxyacetyl nitrate (PAN)
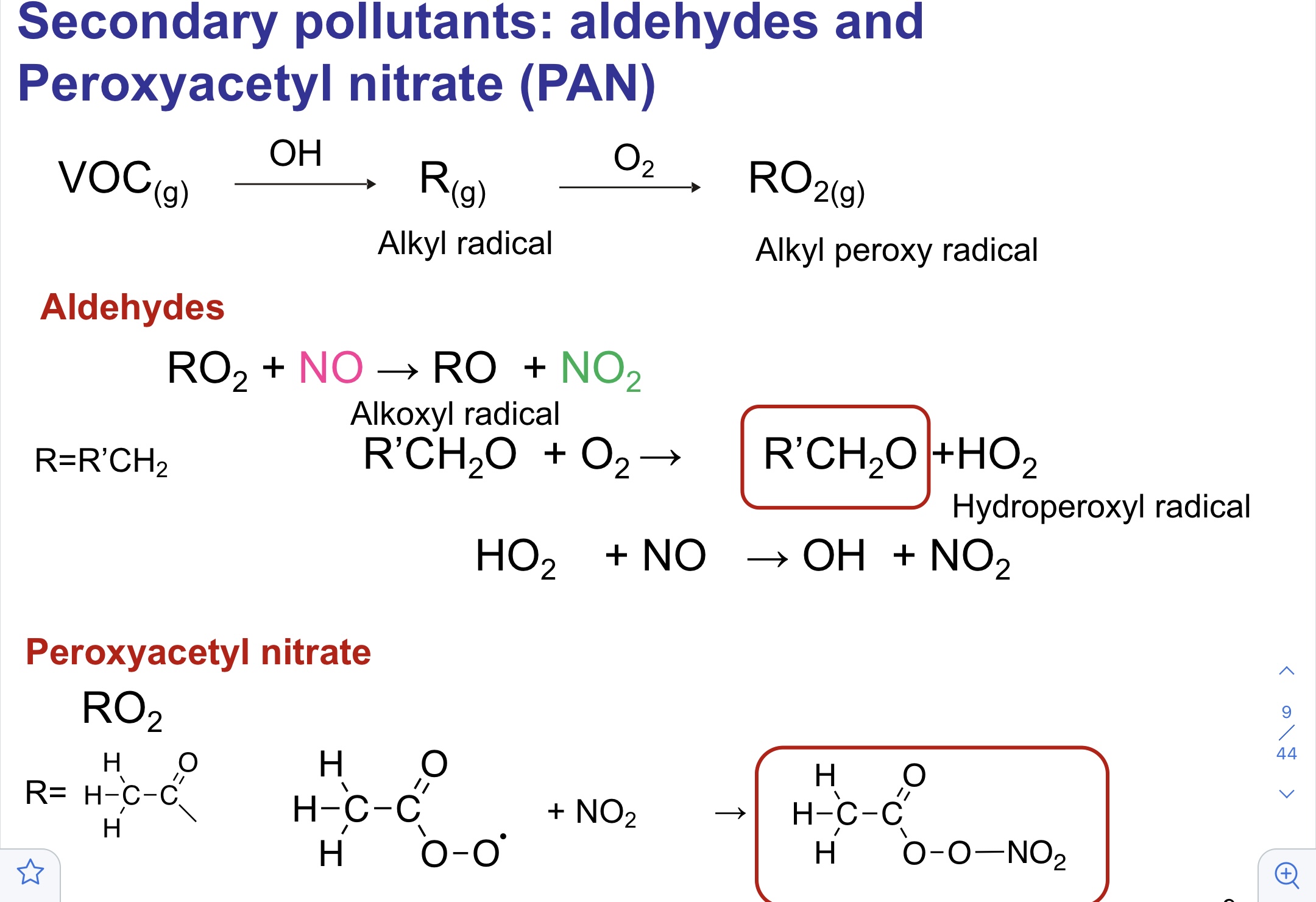
76
New cards
Sources of anthropogenic NOx
combustion engines
77
New cards
Two types of smog
Reducing smog and oxidizing smog ( photochemical smog).
78
New cards
Reducing smog
Largely based on SO2 (London, 1950s) but has disappeared due to emissions regulations
79
New cards
Oxidizing smog.
- Known as photochemical smog, appeared first time in Los Angeles 1940-1950s.
- High levels of ozone.
- can cause eye irritation.
- High levels of ozone.
- can cause eye irritation.
80
New cards
Conditions for photochemical smog.
- warm air (hotter than 18°c )
- significant sunlight (hv)
- hydrocarbons and NOx (many vehicles)
- stable air masses (city surrounded by mountains)
- significant sunlight (hv)
- hydrocarbons and NOx (many vehicles)
- stable air masses (city surrounded by mountains)
81
New cards
Inversion
Hot air above city (stratosphere) which traps the cold smog in the city (troposphere) near the surface of the earth.
82
New cards
Photochemical smog cycle.
- Vehicles exhaust produce VOCs, NO, and NO2 (where NO2 is photolyzed by incoming solar radiation to produce NO, O, O3)
- ozone is low because it is being destroyed O3+No->O2+NO2
- in mid-morning there's lower NO because traffic is lower.
- OH radicals increased sufficiently that VOCs begin to decline.
- 03 concentration begins to increase (low NO concentration)
- around noon, aldehyde production has taken over by free- radicals stock concentration until its consumed
- ozone is low because it is being destroyed O3+No->O2+NO2
- in mid-morning there's lower NO because traffic is lower.
- OH radicals increased sufficiently that VOCs begin to decline.
- 03 concentration begins to increase (low NO concentration)
- around noon, aldehyde production has taken over by free- radicals stock concentration until its consumed
83
New cards
Health effects of ground level ozone.
- Worsening symptoms for people with athsma and other lung diseases, and for people with cardiovascular (heart) disease
- swollen, irritated airways.
- irritation to your eyes, nose, and throat.
- Coughing wheezing
- Headaches
- over time, ozone can cause permanent lung damage.
- swollen, irritated airways.
- irritation to your eyes, nose, and throat.
- Coughing wheezing
- Headaches
- over time, ozone can cause permanent lung damage.
84
New cards
Air qualify health indices.
Low risk: 1-3
Moderate risk: 4-6
High risk: 7-10
Very high risk: Higher than 10
Moderate risk: 4-6
High risk: 7-10
Very high risk: Higher than 10
85
New cards
What can we do to lower ozone levels?
- Lower NOx emissions from automobiles and industry (cars use mixture of 80% N2 / O2 ( ambient air ) as oxidant at high combustion temperatures )
- driving bans and / or temporary shutdown industry/ power plant.
- catalytic converters in vehicles
- lower combustion temperatures.
- re-circulate a fraction of the engine emissions back into flame ( less 02 = less heat)
- driving bans and / or temporary shutdown industry/ power plant.
- catalytic converters in vehicles
- lower combustion temperatures.
- re-circulate a fraction of the engine emissions back into flame ( less 02 = less heat)
86
New cards
Types of Catalytic converters
Two way or three way.
87
New cards
Two way converter
- Completes conversion of CO and other C-containing gases to CO2
2CO+O2->2CO2
CnHm + (n+m/4)O2->nCO2+(m/2)H2O
- air to fuel ratio is a critical parameter
2CO+O2->2CO2
CnHm + (n+m/4)O2->nCO2+(m/2)H2O
- air to fuel ratio is a critical parameter
88
New cards
Three way converter
- Same as two-way plus platinum- rhodium catalyst reduces nitrogen oxides back to N2 and O2
2NO+2H2->N2+2H2O
- H2 and CO are formed during combustion process and act as reducing agents.
- efficiency up to 80-90% when warm (300°c) most emissions come from cold starts
- catalyst also reduces SO2 to H2S (especially when converter is cold)
- air to fuel ratio is a critical parameter
2NO+2H2->N2+2H2O
- H2 and CO are formed during combustion process and act as reducing agents.
- efficiency up to 80-90% when warm (300°c) most emissions come from cold starts
- catalyst also reduces SO2 to H2S (especially when converter is cold)
- air to fuel ratio is a critical parameter
89
New cards
When Catalytic converters are working...
CO and NOx are greatly reduced.
90
New cards
Henry's law, wet deposition
K=[X]aq/[X]g or H =[X]aq/Px
- A solution with [X]aq>HPx is supersaturated and will outgas X
- A solution with [X]aq- A solution with [X]aq~=HPx is at equilibrium; X is still exchanged but no net change in concentration results.
- A solution with [X]aq>HPx is supersaturated and will outgas X
- A solution with [X]aq
91
New cards
Acid rain
precipation that is slightly more acidic than natural unpolluted rain. pH natural ~ 5.6 hover
92
New cards
Two primary drivers of acid rain
SO2 anthropogenic emissions (coal burning, petroleum refining, and smelt processing) and NOx (NOx + H2O -> HNO3)
93
New cards
Predominant acids in acid rain
H2SO4 and HNO3
94
New cards
Major ions in rain
H+, SO4-, NO3-, NH4+
95
New cards
Acidification of droplets by sulfur dioxide.
In polluted air:
- SO2 gas oxidation by molecular oxygen is a very slow process
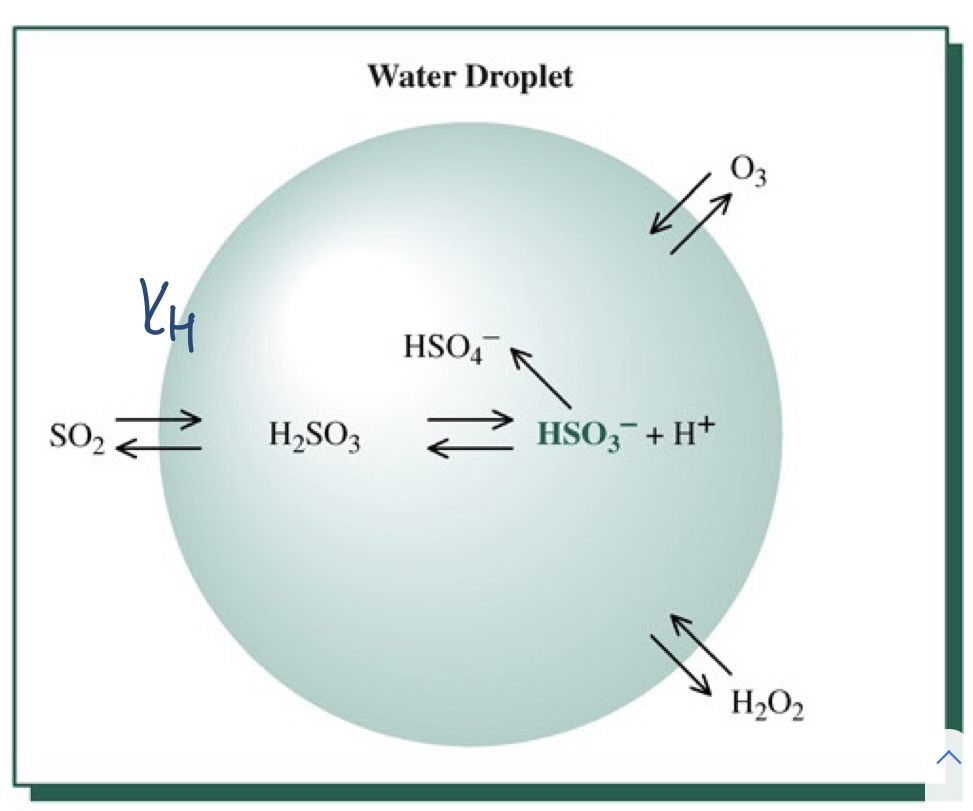
- Research in the early 1980s showed that most of the atmospheric oxidation of SO2 actually takes place in cloud droplets and in the raindrops themselves.
- dissolved aqueous SO2 in airborne droplets is oxidized to H2SO4 by dissolved ozone and hydrogen peroxide.
- SO2 gas oxidation by molecular oxygen is a very slow process
- Research in the early 1980s showed that most of the atmospheric oxidation of SO2 actually takes place in cloud droplets and in the raindrops themselves.
- dissolved aqueous SO2 in airborne droplets is oxidized to H2SO4 by dissolved ozone and hydrogen peroxide.
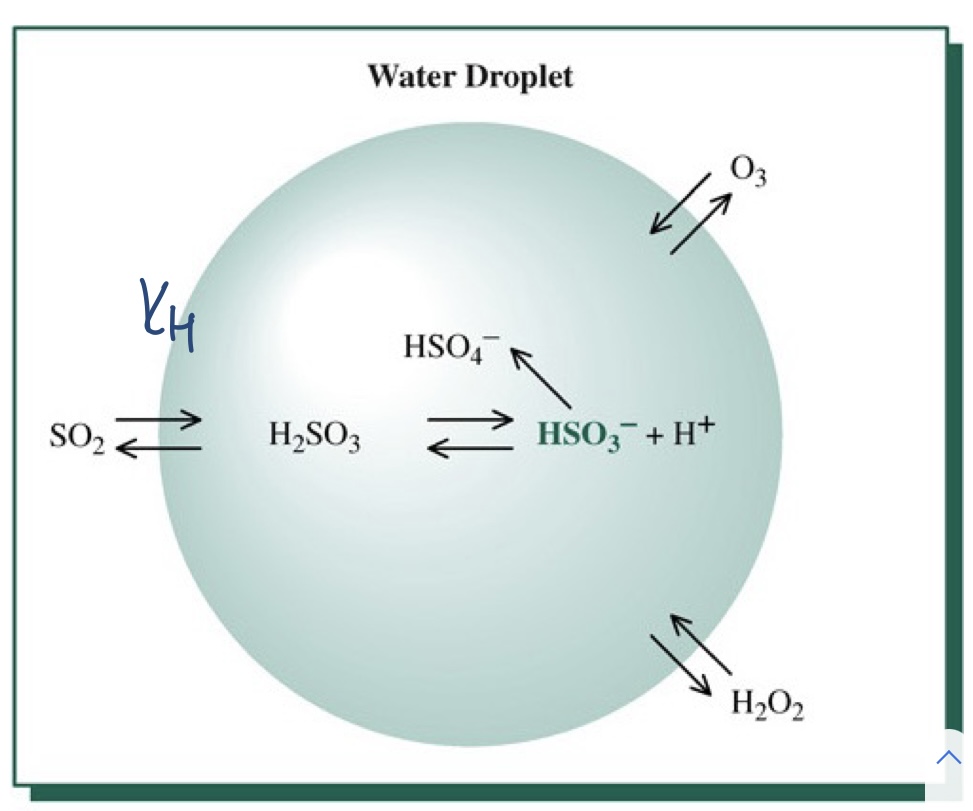
96
New cards
H2SO3 speciation at different pH
- pH 0-2: H2SO3
- pH 2- 7: HSO3-
- pH 7+: SO3-2
- pH 2- 7: HSO3-
- pH 7+: SO3-2
97
New cards
Rain water pH bad areas
Anywhere there is a lot of people like United States, Europe and Canada, mainly, first 2 though. Worse on east coast of US.
98
New cards
Process involved in acid rain
- SO2 and NOx are emitted into the air from human pollution.
- it can be dry dry deposited onto surface from there
-OR it can be oxidized by ozone and turn into acidic versions
- H2SO4 and HNO3 will dissociate in condensation in clouds
- the acidic rain call precipitate (wet deposit)
- it can be dry dry deposited onto surface from there
-OR it can be oxidized by ozone and turn into acidic versions
- H2SO4 and HNO3 will dissociate in condensation in clouds
- the acidic rain call precipitate (wet deposit)
99
New cards
Impacts of acid rain.
- Acid rain can be neutralized by limestone
- it will undergo cation exchange and will remove Ca +2 an important nutrient from soils
- this induces stress to trees, crops, rivers and fish.
- acidified lakes will have high aluminum content ( dissolved from rocks) Al(OH)3 + H+ -> Al+3 +H2O
- acid rain affect regions with granite or quartz bedrock which has little capacity to neutralize acid rain
- SO2 and NOx emissions from tar sands to crude oil in northern Alberta will affect Manitoba and northern Saskatchewan.
- it will undergo cation exchange and will remove Ca +2 an important nutrient from soils
- this induces stress to trees, crops, rivers and fish.
- acidified lakes will have high aluminum content ( dissolved from rocks) Al(OH)3 + H+ -> Al+3 +H2O
- acid rain affect regions with granite or quartz bedrock which has little capacity to neutralize acid rain
- SO2 and NOx emissions from tar sands to crude oil in northern Alberta will affect Manitoba and northern Saskatchewan.
100
New cards
Aerosol
A collection of particulates (solid or liquid) suspended in air
- negligible settling velocity
- small size ( < 100 mm diameter)
- negligible settling velocity
- small size ( < 100 mm diameter)