States of Matter
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
15 Terms
Solid particles
can’t be compressed
vibrate around a fixed position
strong forces of attraction hold the particles close together in a fixed regular arrangement
don’t have much energy
strong forces between molecules
Liquid particles
can’t be compressed
particles still in contact with each other by sliding over each other
weaker forces of attraction between particles
have more energy than particles in a solid
Gas particles
can be compressed
move randomly away from each other
not very dense and collide with the side of a container
weak forces between molecules
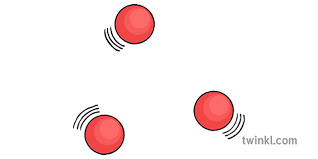
name the particles
Gas
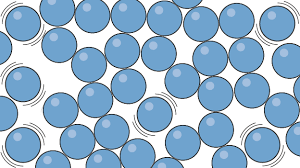
name the particle
liquid
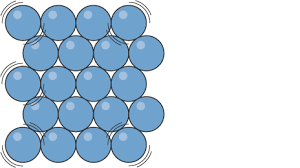
name the particles
solid
Pressure in Gases
Every time the particles hit the side of the container the particles exert a force at right angles on the container- this is called pressure. Pressure= force/area
Measuring density pratically (metal cube)
Measure the length, width and height using a ruler in cm. Times the measurements together to calculate the volume. Put the cube on a mass balance and collect the mass in grams. Divide mass by volume to get density in g/cm3.
Measuring density practically (statue)
Measure the statue on a mass balance to get the mass in grams. Put water into a big enough displacement can so that water starts dripping from the spout. When it stops dripping, place a measuring cylinder under a spout and put the statue in the can. Collect the water that comes from the spout into the cylinder to get the volume. Divide the mass by volume to get the density in g/cm3.
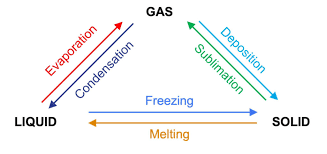
what is this
changes of state
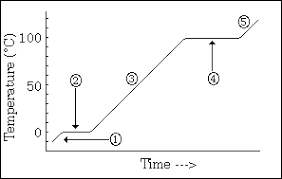
Why is point 2 and 4 flat
It shows a change of state and energy is being used to break the break bonds- potential energy is increasing
What is specific heat capacity 4,200 J/kg degrees in Water
The amount of energy needed to raise the temperature of 1kg of a substance by 1 degrees.
Specific Heat Capacity Practical
Use a mass balance to measure the mass of the insulating container
Fill the container with water and measure its mass again and find the difference of each mass to get the mass of water
Set up the experiment and make sure the joulemeter reads zero
Measure the starting temperature of water, then turn on the power
When the temperature has increases by a certain amount record its temperature increase and the energy on the joulemeter
Calculate the specific heat capacity using the equation and repeat the experiment to get the average specific heat capacity
What is absolute zero
-273 degrees = 0 Kelvin
P1/T1 = P2/T2
T is in Kelvin