General Chemistry II C.10
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
Name a free radical. How do they react with other molecules?
Nitrogen Dioxide (NO2). They react with each other, pairing up their lone electrons
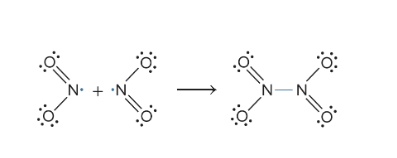
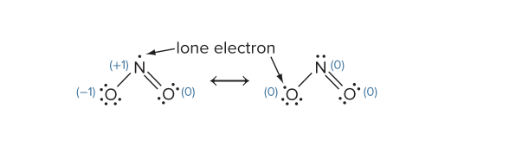
Which resonance structure is preferred based on formula charges?
1. Ones with smaller formal charges
2. The same nonzero formal charges on adjaced atoms are not prefered
3. The more electronegative atom should have a more negative formal charge
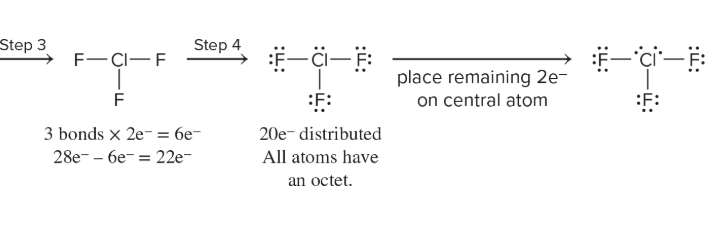
What are expanded valence shells?
Molecules that have more than eight electrons around the central atom
Found third period and below elements with d orbitals
Central atom bonded to more/fewer than four atoms
What is the valence-shell electron-pair repulsion theory (VSPER)?
Each valence electron group around a central atom is located far apart so repulsions between electron group is minimized
Only electron groups around central atom affect shape
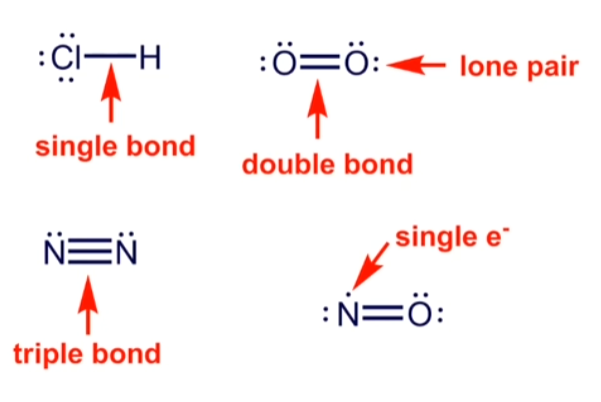
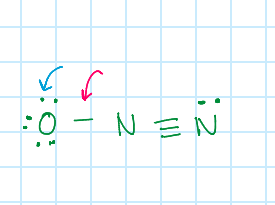
When identifying the shared/unshared valence electrons for a formal charge, which represents the sharing of valence electrons, red or blue?
Red
What is the formula for bond order?
\frac{SharedElectronPairs}{BondedAtomPairs}
What are the five electron-group arrangements
2 electron groups = Linear
3 electron groups- Trigonal planar
4 electron groups= Tetrahedral
5 electron groups= trigonal bipyramidal
6 electron groups= Octahedral
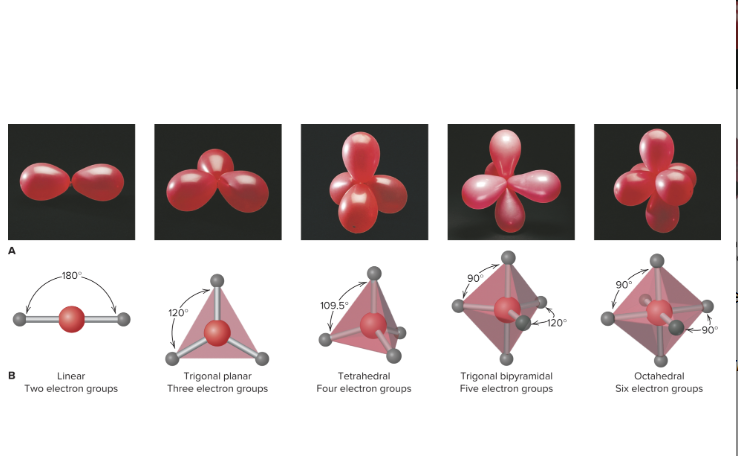
What is the meaning of electron-group arrangement?
Arrangement of shared electron pairs and unshared pairs around central atom
What is the meaning of molecular shape?
3D arrangement of atoms connected by bonding electron groups only
What does A Xm En stand for in classifying molecular shapes?
A= central atom
X= surrounding atoms
E= unshared valence-electron groups (lone pair or lone electron)
True or false? Can you have the same electron-group arrangement and a different molecular shape? Why?
Yes. Unshared electrons around the central atom change the shape of an atom
What does bond angle mean?
Bonds joining nuclei of surrounding atoms to the nucleus of the central atom
AX2 : What is its arrangement and bond angle?
Linear arrangement/shape,180 degrees
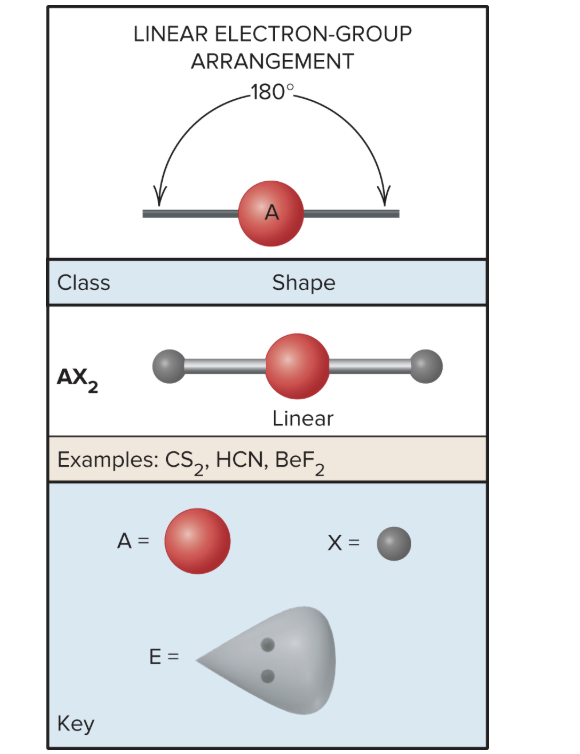
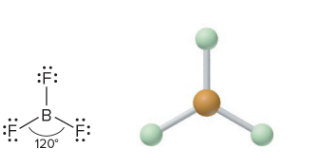
AX3: What is its arrangement and bond angle?
Trigonal planar arrangement/shape, 120 degrees
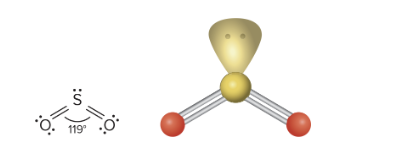
AX2E: What is its arrangement and bond angle?
Trigonal Planar arrangement, bent shape, 120 degrees
How does double bonds affect bond angles?
It repels electrons stronger than in single bonds creating a smaller angle
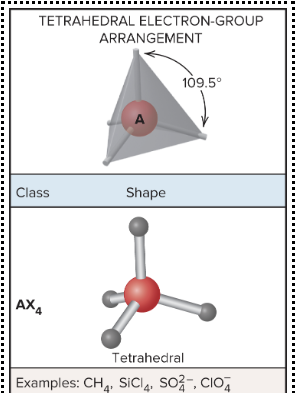
AX4: What is its arrangement and bond angle?
Tetrahedral arrangement, 109.5 bond angle,
What is the formula for formal charge?
Valence electrons - (Bond group + lone electron dots on atom)
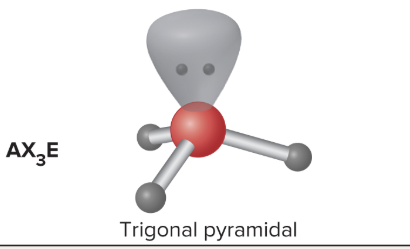
AX3E: What is its arrangement and bond angle?
Trigonal pyramidal shape, >109.5 bond angle
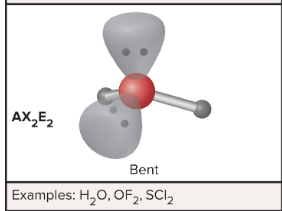
AX2E2: What is its arrangement and bond angle?
Bent shape, > 109.5 degrees
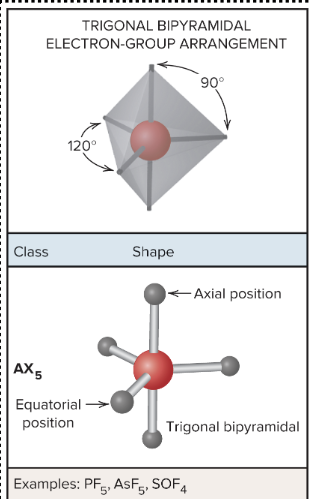
AX5: What is its arrangement and bond angle?
Trigonal Bipyramidal arrangement, 120 bond degree equatorial group and 90-degree axial group
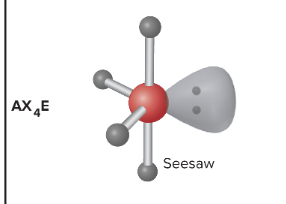
AX4E: What is its arrangement and bond angle?
Seesaw shape, 101.5 equatorial and 86.8 axial degree
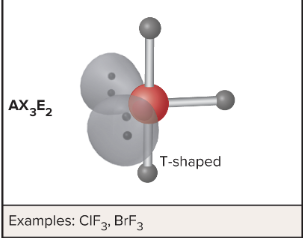
AX3E2: What is its arrangement and bond angle?
T-Shape, small equatorial and 86.2 axial degree
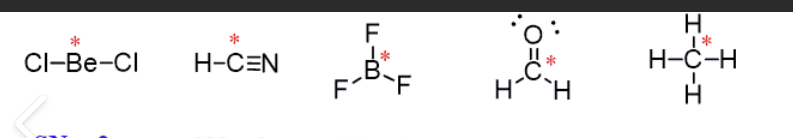
What is a steric number? Identify the steric number for the image.
The amount of atoms bonded to a specific atom + lone pairs surrounding the specific atom.
Steric number = 2
Steric number = 2
Steric number = 3
Steric number = 3
Steric number = 4
True or False: A bond angle of 90 degrees is stronger than of 120 degrees?
True. The greater the angle to weaker the repulsions
AX2E3: What is its arrangement and bond angle?
Linear shape, 180 bond angles,
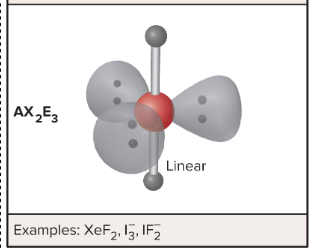
AX6 : What is its arrangement and bond angle
Octahedral arrangement, 90 degree bond angles,
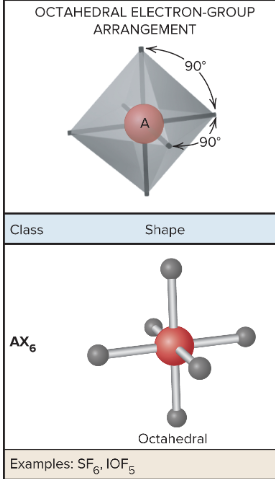
AX5E: What is its arrangement and bond angle?
Square pyramidal, 81.9 bond angle
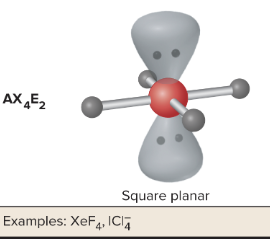
AX4E2: What is its arrangement and bond angle?
Square planar shape, 90 bond angle, AX4E2
What are the steps of using VSEPR to determine molecular shape?
Write Lewis structure
Assign one of the 5 electron-group arrangement
Predict ideal bond angle
Draw and name molecular shape
What is blackbody radiation?
A physical body that absorbs and emits electromagnetic radiation depending on its temperature
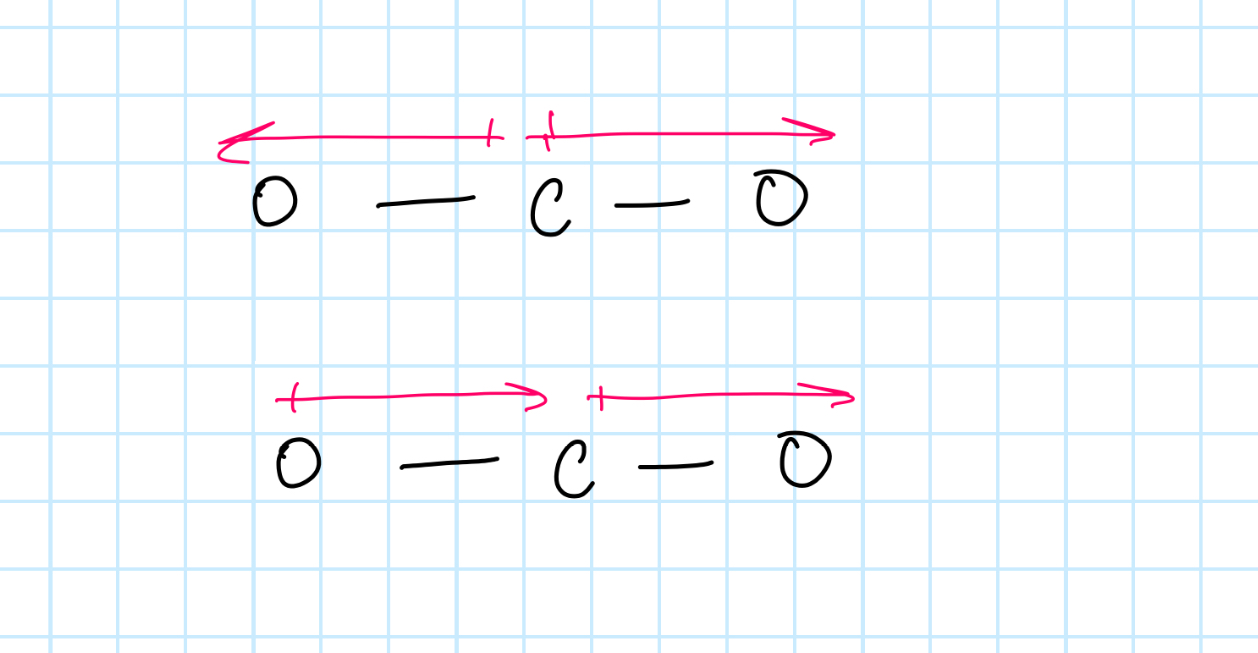
For a molecule to absorb infrared radiation, what happens to its dipole movement? Draw an example of dipole changesusing CO2
Its dipole movement changes.
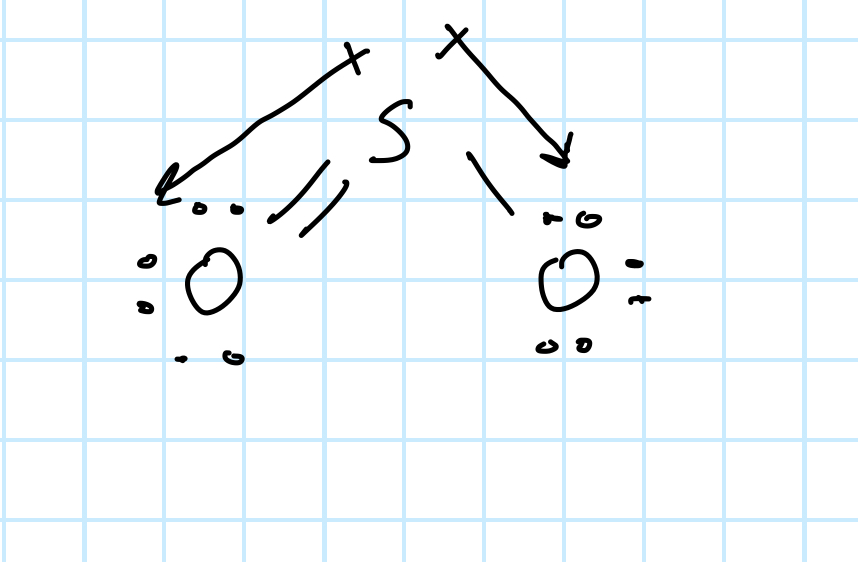
Is the following molecule polar or nonpolar? How do you know?
Polar molecule. Only linear electron arrangements with opposite dipoles cancel out to create a nonpolar bond. In this example the molecule is bent.