Chemistry Q1 Pt. 2
0.0(0)
Card Sorting
1/170
Earn XP
Description and Tags
Last updated 10:34 PM on 12/2/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
171 Terms
1
New cards
Multi-Electron Atoms
- Energies of orbitals depend on n and l, no degenerate states
- Treat multi-electron state as a sum of single-electron states
- Treat multi-electron state as a sum of single-electron states
2
New cards
Shielding
Inner electrons screen/shield outer electrons from the full Coulombic effect of the nucleus, increasing the energy of the orbital where the electron is found
3
New cards
Penetration of the subshells
s > p > d > f
4
New cards
Relative Orbital Energies
- As l increases, the energy of the subshell increases
- All orbitals with the same value of l have the same energy
- All orbitals with the same value of l have the same energy
5
New cards
Electron Configuration
- Electrons occupy lowest orbitals first (Aufbau Principle)
- Each orbital can hold a max of 2 electrons w/ opposite spin (Pauli Exclusion Principle)
- If two or more orbitals have the same energy, electrons will occupy orbitals singly before pairing to reduce electron-electron repulsion and lower atom's energy (Hund's Rule)
- Each orbital can hold a max of 2 electrons w/ opposite spin (Pauli Exclusion Principle)
- If two or more orbitals have the same energy, electrons will occupy orbitals singly before pairing to reduce electron-electron repulsion and lower atom's energy (Hund's Rule)
6
New cards
Two Types of Representations
- spdf notation: write shell/subshell in increasing energy, # of electrons in superscript (e. 1s^2 2s^2)
- Orbital Diagrams
- Orbital Diagrams
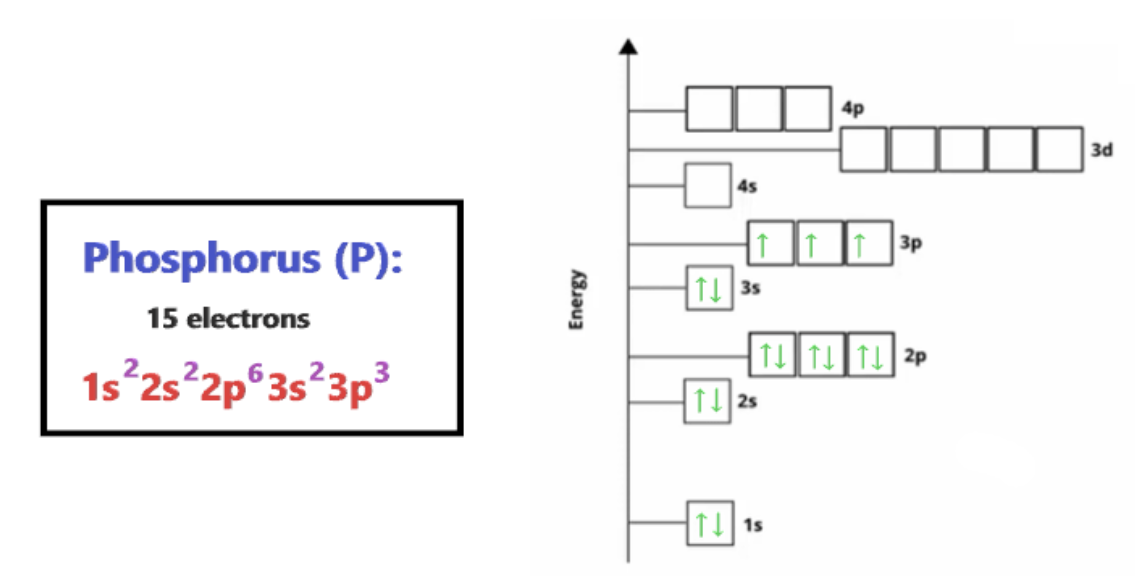
7
New cards
Electron Configurations - Period 1
- Hydrogen, Z = 1, 1s^1
- Helium, Z = 2, Fill the first shell to create a CLOSED SHELL, 1s^2
- Helium, Z = 2, Fill the first shell to create a CLOSED SHELL, 1s^2
8
New cards
Electron Configurations - Period 2
- Lithium, Z = 3, [He]2s^1
And so on and so forth
And so on and so forth
9
New cards
Noble Gases in Notation
- Chemically inert closed shells that are full
10
New cards
Electron Configuration - Period 4
- 4s subshell is lower in energy than the 3d subshell so it fills first
- 4s < 3d < 4p < 5s < 4d < 5p
- 4s < 3d < 4p < 5s < 4d < 5p
11
New cards
The (n + l) Rule
The ordering of the energies of the orbitals in a multi-electron atom increases with the value of n + 1
12
New cards
Valence Shell
Outermost, highest energy shell, equal to the row # of the element
13
New cards
Valence Electrons
- Determine element's physical/chemical properties
- # of valence electrons = last digit of group #
- # of valence electrons = last digit of group #
14
New cards
Excited State
Atom absorbs energy, promoting an electron to a higher energy orbital
- Ground state of C: [He]2s^(2) 2p^(2)
Excited state of C: [He]2s^(2) 2p^(3)
- Ground state of C: [He]2s^(2) 2p^(2)
Excited state of C: [He]2s^(2) 2p^(3)
15
New cards
Effective Nuclear Charge (Zeff)
- Net positive charge attracting an electron in an atom
- Zeff is always less than Z in a multi-electron atom because of the shielding effect
- Zeff is always less than Z in a multi-electron atom because of the shielding effect
16
New cards
Zeff Equation
Zeff = Z (actual nuclear charge) - S (core electrons)
17
New cards
Across a period, Zeff...
Increases as electrons join the same valence shell
18
New cards
Down a group, Zeff...
Decreases
19
New cards
Atomic Radius
- Estimated as half the distance (d/2) between the center of neighboring nuclei
- 2 metal atoms: Metallic radius
- 2 nonmetal atoms: Covalent radius
- 2 gaseous atoms: Van der Waals radius
- 2 metal atoms: Metallic radius
- 2 nonmetal atoms: Covalent radius
- 2 gaseous atoms: Van der Waals radius
20
New cards
Ionic Radius
Effective radius of an ion in an ionic solid
21
New cards
Across a period, atomic radii...
Decreases as more electrons are attracted to more protons int he nucleus
22
New cards
Down a group, atomic radii...
Increases
23
New cards
Cation Size
Always SMALLER than the atoms they form from
24
New cards
Isoelectronic Cations
The more positive the ionic charge, the smaller the cation
25
New cards
Anion Size
Always LARGER than the atoms they form from
26
New cards
Isoelectronic Anions
The more negative the ionic charge, the larger the anion
27
New cards
Ionization Energy (IE)
- Minimum energy required to remove one electron from a single atom in the gaseous state
- The larger the IE, the more stable the electronic configuration of the atom
- The larger the IE, the more stable the electronic configuration of the atom
28
New cards
Across a period, IE...
Increases due to increased force of attraction between nucleus and electrons
29
New cards
Down a group, IE...
Decreases due to an increasing n
30
New cards
IE1 < IE2 < IE3
Atoms are less stable after electron loss, more energy is required to remove more electrons for higher IEs
31
New cards
Electron Affinity (EA)
- Energy released on adding one electron to an atom in the gaseous state
- The larger the EA, the more stable the anion that is formed
- The larger the EA, the more stable the anion that is formed
32
New cards
Across a period, EA...
Increases
33
New cards
Down a group, EA...
Decreases
34
New cards
Electron Affinity (EA1 vs. EA2)
ANS: Adding a second electron (EA2) always requires energy input because of the electrostatic repulsions so EA2 will be negative
35
New cards
Down a group, reactivity...
increases as shielding lowers nuclear pull on electrons
36
New cards
Across a period, reactivity...
decreases
37
New cards
Down a group, lattice energy...
Decreases for molecules
38
New cards
Diagonal Relationship in Main Group
- Some pairs of elements diagonally-adjacent to each other have similar chemical properties
- Atomic radii decreases from left to right, increase top to bottom
- IE increases from left to right but decreases from top to bottom
- Atomic radii decreases from left to right, increase top to bottom
- IE increases from left to right but decreases from top to bottom
39
New cards
Diamagnetism
- Atoms/ions which possess only spin-paired electrons
- Magnetic effects cancel out and they are weakly repelled by magnetic field
- Magnetic effects cancel out and they are weakly repelled by magnetic field
40
New cards
Paramagnetism
- Atoms/ions which possess one or more orbitally unpaired electrons
- Attraction to magnets
- Attraction to magnets
41
New cards
Chemical Bond
Result of attraction between two or more atoms/ions that involves sharing or transferring electrons
42
New cards
Chemical Bond Determinants
Ionization Energy and Electron Affinity: How readily an atom gives up/accepts an electron
43
New cards
Why do they form?
- Reduction in the Total Energy
- Lowering of the potential energy (Potential Energy of bonded atoms must be lower than that of non-bonded atoms)
- Lowering of the potential energy (Potential Energy of bonded atoms must be lower than that of non-bonded atoms)
44
New cards
Maximum Stability for an Atom
- Atom is isoelectronic with a noble gas atom, reaches a stable electron configuration
45
New cards
Lewis Dot Structure
- Dots (denote valence electrons) are distributed around the four sides of an element symbol, first singularly and then in pairs
- Combine Lewis structures to represent transfer/sharing of electrons in an ionic or covalent bond
- Most atoms gain/lose atoms to complete octet or duplet
- Combine Lewis structures to represent transfer/sharing of electrons in an ionic or covalent bond
- Most atoms gain/lose atoms to complete octet or duplet
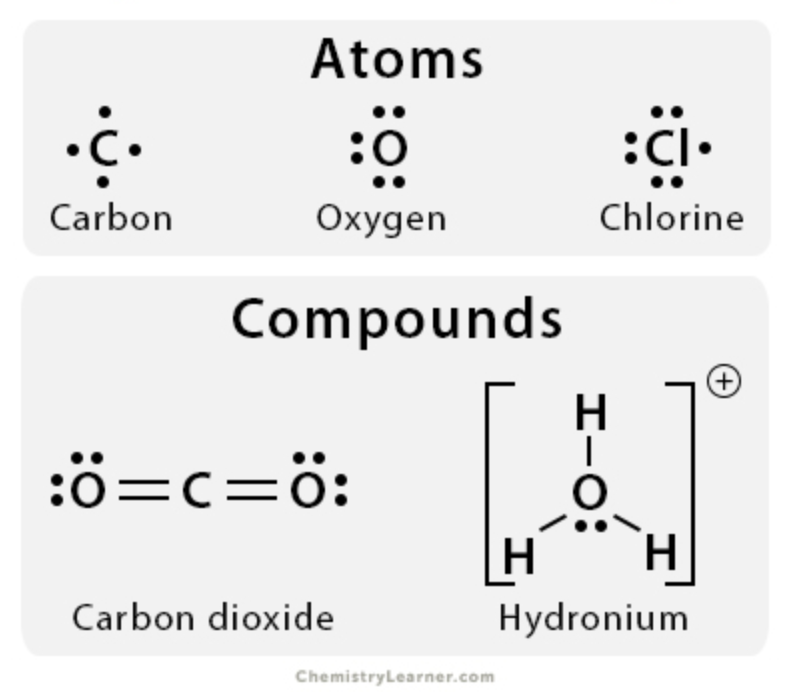
46
New cards
Chemical Formula
- Composition: What numbers/kinds of atoms present?
- Constitution: How are the atoms connected?
- Configuration: How are the atoms positioned in 3-D space?
- Constitution: How are the atoms connected?
- Configuration: How are the atoms positioned in 3-D space?
47
New cards
Empirical Formula
Shows relative # and kinds of atoms present
Ex. CH2O
Ex. CH2O
48
New cards
Molecular Formula
Shows actual # and kinds of atoms present
Ex. C2H4O2
Ex. C2H4O2
49
New cards
Structural Formula
Shows the atom connectivity with #/kinds of atoms present
50
New cards
Ionic Bonding
- Occurs between ions (a cation and anion)
- Cation is a metal, Anion is a non-metal
- Compounds represented by empirical formula ONLY, termed "formula unit"
- Cation is a metal, Anion is a non-metal
- Compounds represented by empirical formula ONLY, termed "formula unit"
51
New cards
Step 1 of Ionic Bonding
- Form Ions
- Metals tend to lose electrons, Non-metals tend to gain them
- Main Group Elements (Groups 14/15) don't have a strong preference for either gaining or losing electrons
- Transition Metal Cations firs lose valence s electrons, may lose d electrons (2+ is common)
- Cations have electron configuration of preceding Noble Gas
- Anions have electron configuration of next Noble Gas
- Metals tend to lose electrons, Non-metals tend to gain them
- Main Group Elements (Groups 14/15) don't have a strong preference for either gaining or losing electrons
- Transition Metal Cations firs lose valence s electrons, may lose d electrons (2+ is common)
- Cations have electron configuration of preceding Noble Gas
- Anions have electron configuration of next Noble Gas
52
New cards
Inert-Pair Effect in Lower P-Block Elements
- Cation formed by loss of ONLY the p electrons
- p-Block of periods 4, 5, or 6 formt wo types of cations depending on if they lose only the p subshell or both p and s subshell
- p-Block of periods 4, 5, or 6 formt wo types of cations depending on if they lose only the p subshell or both p and s subshell
53
New cards
Step 2 of Ionic Bonding
- Combine Ions
- Ionic Bond is complete transfer of one or more electrons, held together by electrostatic forces
- Ionic Compound has lowest ratio of ions
- Ionic Bond is complete transfer of one or more electrons, held together by electrostatic forces
- Ionic Compound has lowest ratio of ions
54
New cards
Chemical Formula of Ionic Compounds
- Cations listed first,t hen anions second
- Eliminate common factor in subscripts
- Simplest ratio of anions and cations equal zero charge
- Eliminate common factor in subscripts
- Simplest ratio of anions and cations equal zero charge
55
New cards
Formula Mass (amu)
Sum of atomic mass of each atom x # of atoms of that element int he compound
56
New cards
How do we name Ions and Ionic Compounds?
- Depends on if the ions are monoatomic or polyatomic
57
New cards
Metal cations (Main Groups 1, 2, and 13)
Identify metal and add "ion" to the end
Ex. K+ = Potassium Ion
Ex. K+ = Potassium Ion
58
New cards
Transition Metals/Metals (Groups 13-15)
Use Roman numerals to indicate ion's charge
Ex. Mercury = Hg2^2+ = Mercury (I)
Mercury = Hg2+ = Mercury (II)
Ex. Mercury = Hg2^2+ = Mercury (I)
Mercury = Hg2+ = Mercury (II)
59
New cards
Anions
End with "ide" and "ion"
Ex. Bromide = Br- = Bromide ion
Ex. Bromide = Br- = Bromide ion
60
New cards
Polyatomic Ions
Ions composed of more than one atom (covalently bonded)
- Positive Molecular Ions: End in "ium" or "onium"
Ex. NH4^1+ = Ammonium
- Negative Molecular Ions: End in "ide", "ite", or "ate"
Ex. ClO^1- = Chloride ClO2^1- = Chlorite ClO3^1- = Chlorate
- Positive Molecular Ions: End in "ium" or "onium"
Ex. NH4^1+ = Ammonium
- Negative Molecular Ions: End in "ide", "ite", or "ate"
Ex. ClO^1- = Chloride ClO2^1- = Chlorite ClO3^1- = Chlorate
61
New cards
3-D Lattices of Ions
- Ion sizes/charges determine crystalline structures
62
New cards
Ionic Bonding: The Energetics
- Ion-Pair of from gas phase
- Step 1: Ionization of Sodium (loses electron); Ionization Energy is given
- Step 2: Electron Gain by Chlorine; Electron Affinity is given
- Step 3: Formation of the ion-pair into NaCl; Apply Coulomb's Law by multiplying charges of two ions and dividing by distance (radii of ions given, add together to get total distance)
Net Charge = E = (IE - EA) - NaCl Energy from Coulomb's law
- Step 1: Ionization of Sodium (loses electron); Ionization Energy is given
- Step 2: Electron Gain by Chlorine; Electron Affinity is given
- Step 3: Formation of the ion-pair into NaCl; Apply Coulomb's Law by multiplying charges of two ions and dividing by distance (radii of ions given, add together to get total distance)
Net Charge = E = (IE - EA) - NaCl Energy from Coulomb's law
63
New cards
Born-Haber Cycle
Features a series of (hypothetical) steps starting from the elements in their standard states, used to calculate Lattice Energies
64
New cards
Lattice Energy Trends
The greatest stabilization in the solid occurs when ions are small and highly charged
65
New cards
How Can We Explain the Conductivity of Salt Solutions?
Solid = No conductivity
Molten = Some conductivity
Dissolved in water = Lots of conductivity
Molten = Some conductivity
Dissolved in water = Lots of conductivity
66
New cards
What Import do Ionic Compounds Have in Our Lives?
Real-world uses and examples of Ionic Compounds
67
New cards
Stalagmites/Stalactites
Calcium carbonate/Magnesium Carbonate
68
New cards
Bones and Cement Matrices
Calcium Phosphate used to replace damaged/diseased bone
69
New cards
Fertilizers
Ammonium Nitrate
70
New cards
Cleaners/Disinfectants
Solution of Sodium Hypochlorite
71
New cards
Baking and Food Additives
Sodium Bicarbonate (creates CO2 gas in baking)
Sodium Nitrite (food additive)
Sodium Nitrite (food additive)
72
New cards
Multivitamin Supplements
Magnesium oxide, Potassium Chloride, Calcium Carbonate, Ferrous Fumarate, Calcium Stearate, Manganese Sulfate, Nickelous Sulfate, Potassium Iodide, Sodium Ascorbate, Sodium Benzoate, Sodium Citrate, Titanium Dioxide, Zinc Oxide
73
New cards
Covalent Bonding
- Create molecules, electrically neutral groups held together by covalent bonds
- Occurs between non-metals/metalloids
- Occurs between non-metals/metalloids
74
New cards
Lewis Structure of Covalent Bond
- Sharing electrons between atoms
- Two dots or a line represent a single bond, or shared pair of electrons
- Two dots or a line represent a single bond, or shared pair of electrons
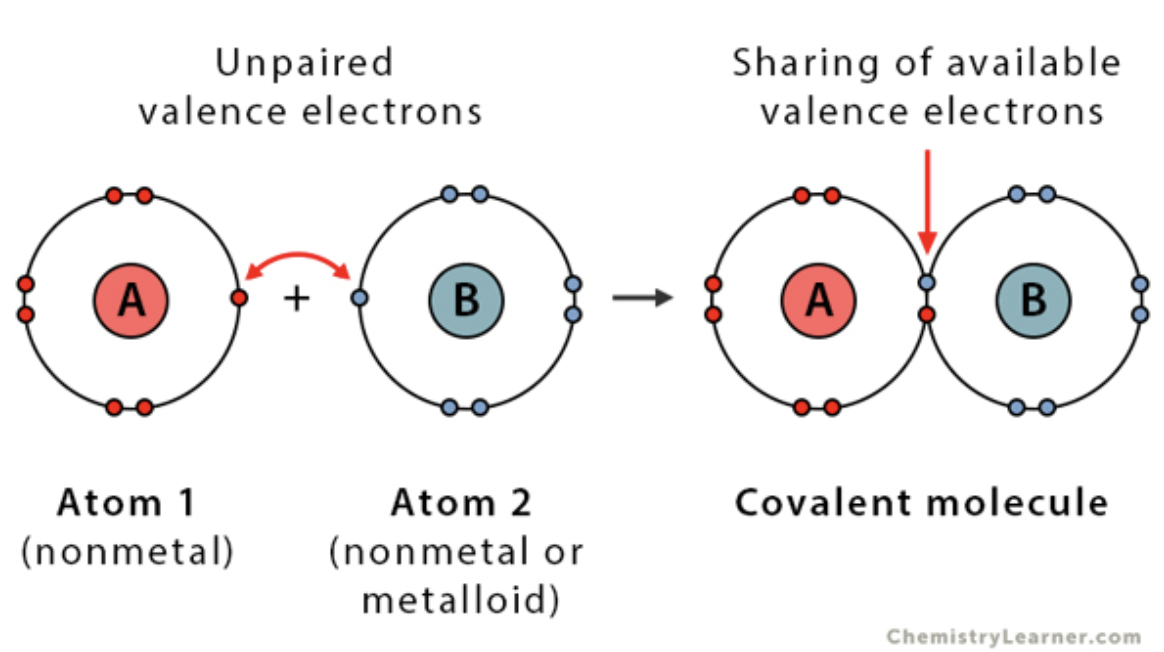
75
New cards
How do Covalent Bonds Form?
- As two atoms with incomplete valence shells interact, their electron clouds overlap and concentrates the electron density, forming a covalent bond
- If atoms are too close, strong repulsions occur
- If atoms are too far apart, attractions are weak and no bonding occurs
- When atoms are optimally separated, the energy is at a minimum and the distance between the nuclei is the bond length
- If atoms are too close, strong repulsions occur
- If atoms are too far apart, attractions are weak and no bonding occurs
- When atoms are optimally separated, the energy is at a minimum and the distance between the nuclei is the bond length
76
New cards
Diatomic Elements: Period 2
- OCTET RULE applies for C, N, O and F
- Multiple Bonds, or more than one shared pair of electrons, can form
Ex. Single, Double or Triple Covalent Bonds
- Bond Order (B.O.) indicates # of shared electron pairs between atoms
- Multiple Bonds, or more than one shared pair of electrons, can form
Ex. Single, Double or Triple Covalent Bonds
- Bond Order (B.O.) indicates # of shared electron pairs between atoms
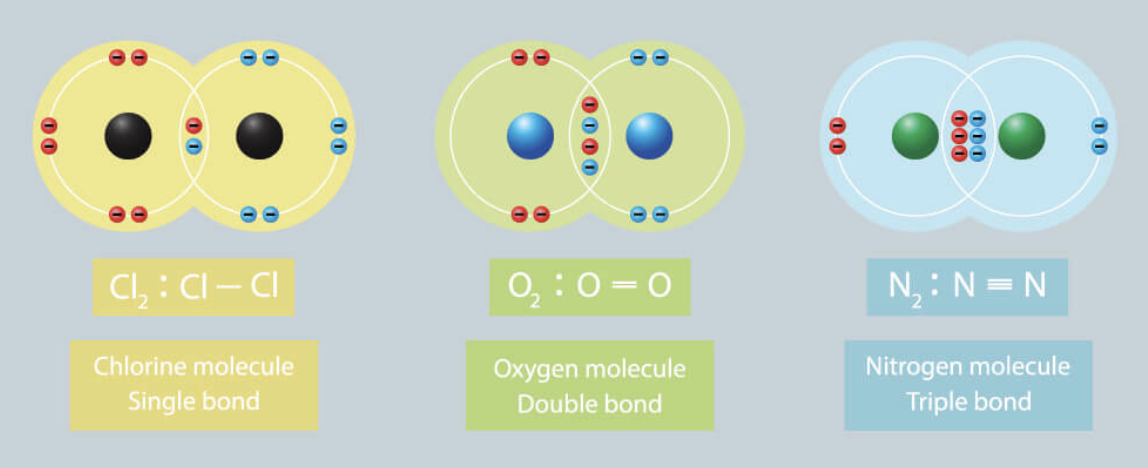
77
New cards
Chemical Formulae: Covalent Compounds
- Element of lowest IE is written first
- Write names with numerical prefixes (don't add mono- to first element if there is only one present in compound)
- Ex. N2O5 = Dinitrogen Pentoxide
- Write names with numerical prefixes (don't add mono- to first element if there is only one present in compound)
- Ex. N2O5 = Dinitrogen Pentoxide
78
New cards
Numerical Prefixes (1-10)
Mono
Di
Tri
Tetra
Penta
Hexa
Hepta
Octa
Nona
Deca
Di
Tri
Tetra
Penta
Hexa
Hepta
Octa
Nona
Deca
79
New cards
Formal Charge
FC = # of valence electrons - # of bonds made - # of unshared electrons
80
New cards
Formal Charge Rules
- Sum of Formal Charges should equal the overall charge of the atom/ion
- Negative Formal Charges go to atoms with highest IE
- Positive Formal Charges go to atoms with lowest IE
- Negative Formal Charges go to atoms with highest IE
- Positive Formal Charges go to atoms with lowest IE
81
New cards
Resonance: Equivalent Structures
- When certain molecules/ions have 2 or more valid but different Lewis structures
82
New cards
Resonance Hybrid
Combines structures to make a more accurate description of the real molecular structure
83
New cards
Bond Order
B.O. = (Add up bond types)/# of bonds made
Ex. O=O-O (2 + 1)/2 = 1.5
Ex. O=O-O (2 + 1)/2 = 1.5
84
New cards
Resonance: Inequivalent Structures
- When there are several (inequivalent) resonance structures that do no contribute equally to resonance hybrid
- Sometimes one structure is the main contributor
- Consider if there is a single bond between central and terminal atoms
- Consider having low formal charges, positive FC on low IE and negative FC on high IE
- Sometimes one structure is the main contributor
- Consider if there is a single bond between central and terminal atoms
- Consider having low formal charges, positive FC on low IE and negative FC on high IE
85
New cards
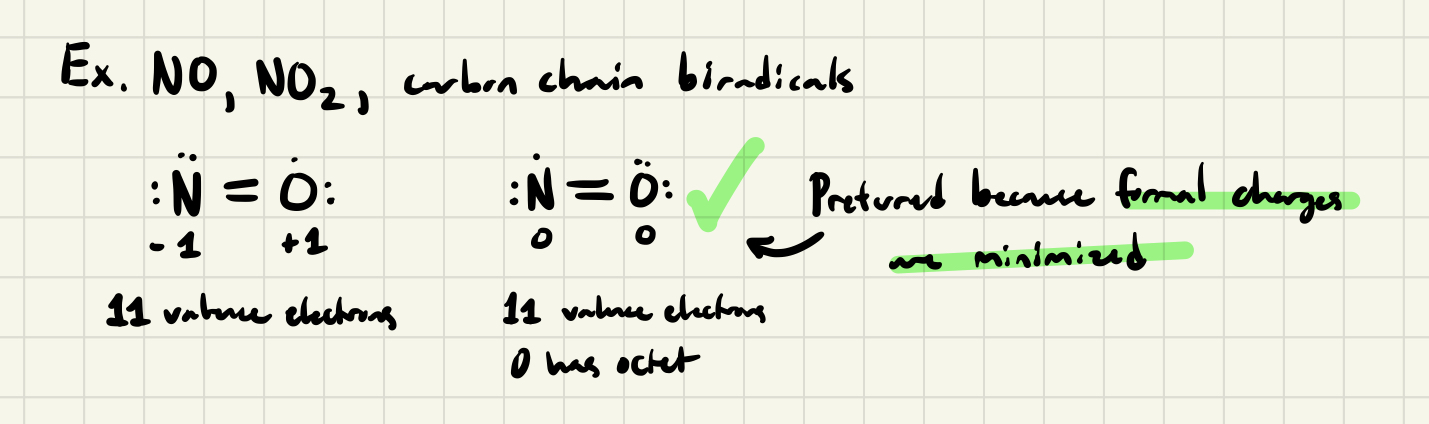
Odd-Electron Molecules (Radicals)
- If a molecule has an odd # of electrons, it's not possible to write a Lewis structure in which there is an octet of electrons about each atom
- Valid Lewis structures have: Maximized the # of atoms which possess an octet and place the odd electron on the atom w/ lowest IE (minimize formal charges)
- Valid Lewis structures have: Maximized the # of atoms which possess an octet and place the odd electron on the atom w/ lowest IE (minimize formal charges)
86
New cards
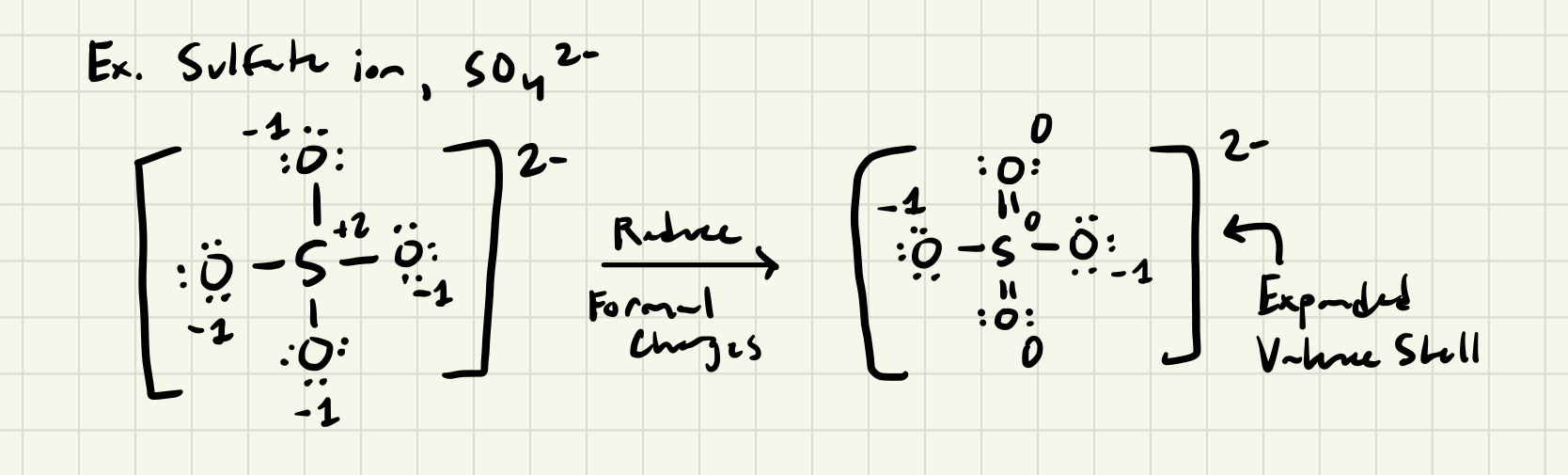
Hypervalent (Valence Shell Expanded) Molecules
- Some atoms in the 3rd period and beyond appear to accommodate more than 4 pairs of electrons, suggesting expanded valence shells
- Valid Lewis Structures first assign lone pairs to the outer atoms to give them octets, then assigning remaining valence electrons to the central atom as lone pairs
- Valid Lewis Structures first assign lone pairs to the outer atoms to give them octets, then assigning remaining valence electrons to the central atom as lone pairs
87
New cards
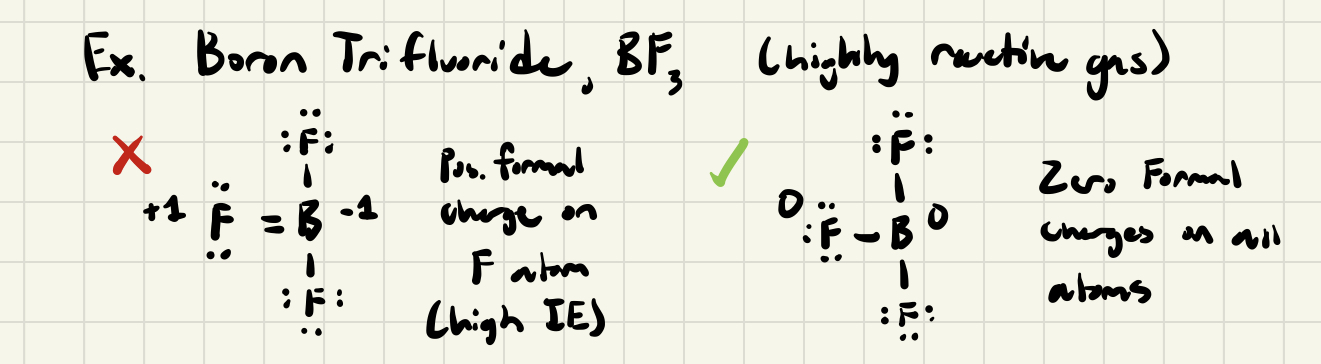
Hypovalent (Octet-Deficient) Molecules
- If molecule possess less than the # of required valence electrons (electron deficient), it won't be possible to write a Lewis structure in which there is an octet of electrons about each atom
- Valid Lewis Structures assign octets to the atoms with highest IE, then incomplete octet occurs on the least electronegative atom
- Valid Lewis Structures assign octets to the atoms with highest IE, then incomplete octet occurs on the least electronegative atom
88
New cards
Properties of the Covalent Bond: Bond Strength
- Measured by Dissociation Energy (D), kJ mol^-1
- Energy required to separate gaseous bonded atoms; deeper the potential energy well, the stronger the bond
- Energy required to separate gaseous bonded atoms; deeper the potential energy well, the stronger the bond
89
New cards
Properties of the Covalent Bond: Bond Length
- Distance between atomic nuclei in a given covalent bond
- Measured experimentally; as bond length decreases, bond strength increases
- Measured experimentally; as bond length decreases, bond strength increases
90
New cards
Trends: Bond Orders
When more electrons are shared between two nuclei, bond length decreases and bond strength increases
91
New cards
Non-Polar Covalent Bond
- Typically connect identical atoms, electrons are shared equally
92
New cards
Polar Covalent Bond
- Connect atoms of different elements
- Nucleus of one atom attracts electrons more strongly than the nucleus of the other atom, acquiring a partial negative charge and leaving the other atom with a partial positive charge
- Electric Dipole Moment: Arrow points from - to +
- Calculated by Q (charge) x r (distance m)
- Nucleus of one atom attracts electrons more strongly than the nucleus of the other atom, acquiring a partial negative charge and leaving the other atom with a partial positive charge
- Electric Dipole Moment: Arrow points from - to +
- Calculated by Q (charge) x r (distance m)
93
New cards
Correcting the Covalent Model: Electronegativity
- Measures the ability/tendency of an atom to attract electrons from another atom to which it is bonded to
- The element with the higher E.N. value will attract electrons more strongly in the bond (greater electron-pulling power)
- The element with the higher E.N. value will attract electrons more strongly in the bond (greater electron-pulling power)
94
New cards
Electronegativity: Mulliken Definition
- Use Ionization Energy and Electron Affinity provide indication of how readily an atom may give up or accept an electron
- Higher the IE, the greater the value of E.N.
- E.N. = 1/2(IE + EA)
- Higher the IE, the greater the value of E.N.
- E.N. = 1/2(IE + EA)
95
New cards
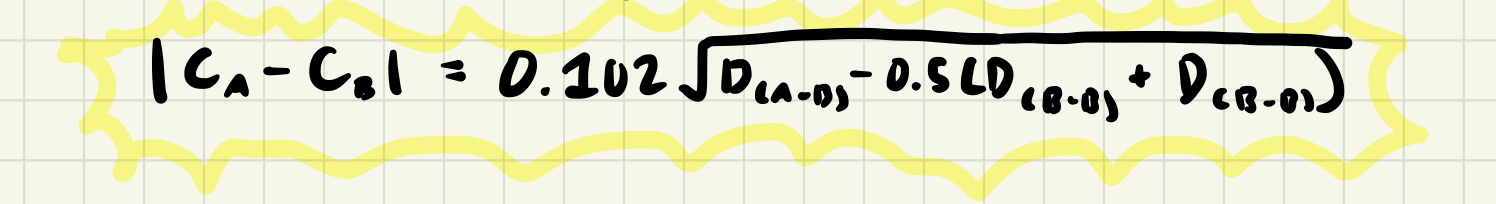
Electronegativity: Pauling Definition
- Created an electronegativity scale based on dissociation energies, D in eV, of the homonuclear and heteronuclear bonds
- Described the difference in electronegativities between two elements
- Excess bond energy is a result of the ionic component of the bond caused by partial charges
- Described the difference in electronegativities between two elements
- Excess bond energy is a result of the ionic component of the bond caused by partial charges
96
New cards
Electronegativity Differences as a Measure
- Polarity of a covalent bond is directly proportional to the difference in electronegativity of the bonded atoms
- As E.N. increases, so do the partial charges on each atom, resulting in a bond with a greater ionic character
- As E.N. increases, so do the partial charges on each atom, resulting in a bond with a greater ionic character

97
New cards
Covalent E.N. Range
0 - 0.3
98
New cards
Polar Covalent E.N. Range
0.4 - 2.0
99
New cards
Ionic E.N. Range
2.1+
100
New cards
3 Different Ways of Depicting Molecular Shape
- Dashed-Wedged Line Formula
- Ball-and-Stick Model
- Space-Filling Model
- Ball-and-Stick Model
- Space-Filling Model