Ch 20/21 Reactants
0.0(0)
Card Sorting
1/11
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
1
New cards
Reduction to alditol
NaBH4, H3O+
2
New cards
Oxidation to aldonic acids (ketoses/aldoses)
-OH, Ag+, NH3
3
New cards
oxidation to aldaric acid (aldehydes and primary alcohols)
HNO3/Heat
4
New cards
Lengthening (Kiliani-Fischer Synthesis)
-CN, HCl, H2/Lindlar’s catalyst
5
New cards
Shortening (Wohl Degradation)
NH2OH/trace acid, Ac2O 100C, -OH/H2O
6
New cards
AA synthesis - HVZ and ammonia
1. Br2/PBr3 2. H2O, excess NH3
7
New cards
AA synthesis - reductive amination
alpha-keto acid. 1. NH3 (excess), trace acid 2. H2, Pd/C
8
New cards
N-phthalimidomalonic ester synthesis
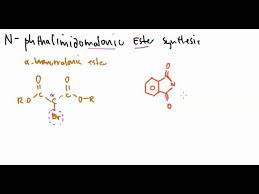
forms tetrahedral → AA
9
New cards
Strecker Synthesis (aldehyde → imine)
NH3/trace acid, -CN, HCl/H2O/Heat
10
New cards
disulfide bonds
-OH/H2O, Br2
11
New cards
Amino protecting group
t-Boc, remove with TFA/CH3Cl2
12
New cards
carboxyl activating group
DCC, remove with HF