c343 final
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
73 Terms
organic chemistry reaction parts
1. reaction
2. separation
3. purification
4. analysis
ex 1 summary
- simple acid base reaction: proton transfer converts the base into a solid white precipitate
- cold water maximizes product recovery
ex 1 procedure
1. dissolved sodium benzoate in DI water and then used HCl to bring the pH down to 2
2. put the solution in an ice bath
3. vacuum filtered the product and washed with cold DI water
ex 1: why did we bring the pH down to 2?
to precipitate as much of the sodium benzoate that we could
ex 1: what did we put the solution in an ice bath?
to maximize product recovery
ex 1: which of these species is a stronger/weaker acid: HCl and benzoic acid?
stronger: HCl
weaker: benzoic acid
ex 1: which side of the reaction does equilibrium lie?
towards the products because HCl is more acidic than benzoic acid and equilibrium favors the formation of a weaker acid
ex 1: what would happen if you used H2SO4 instead of HCl to bring down the pH to 2?
no effect
ex 1: what would happen if you used room temp water to wash your filtrate?
room temp water might lead to some of the desired product dissolving and being lost
ex 1: what would you expect to see if you didn't bring the pH down to 2?
not as much of the sodium benzoate would precipitate
ex 1: you failed to stir the solution while adding the HCl. your pH paper indicates that you are at 2 but you have no precipitate. how do you explain this?
the top of the solution is 2, but the rest of the solution is not, which is why there is no precipitate
ex 2 summary
taking advantage of each components differing solubilities to separate them from each other
ex 2: important solubilities to know
- sucrose is soluble in water but insoluble in DCM
- ASA and unknown are both soluble in DCM
- acetylsalicylate (ASA conjugate base) is water soluble
ex 2 procedure
1. dissolve 3 grams of panacetin in DCM and filter the sucrose
2. place the filtrate into a separatory funnel and add NaOH solution, mix both layers
3. separate the layers
4. add another portion of NaOH solution and separate again
5. add dropwise HCl to the aqueous solution, place in ice bath, and vacuum filter the aspirin
6. place the DCM solution in a round-bottom flask and distill off the DCM, collect your unknown
ex 2: why do we mix both layers thoroughly?
insufficient separation would leave ASA in the organic layer
ex 2: when separating, why do we get rid of the bottom layer out of the bottom, and the top layer out of the top?
if both liquids exit through the bottom of the funnel, cross contamination of the two solutions could occur
ex 2: how much solid NaOH to make a 50 mL NaOH solution at 1M concentration?
1M NaOH x 0.05 L = 0.05 mol NaOH x 39.997 g/mol = 2g NaOH
2g NaOH (s)
ex 2: how much concentrated HCl (12M) to make 15 mL of 6M HCl?
(12M HCl)(V2) = (6M HCl)(0.015L)
V2 = 0.0075 L
0.0075 L HCl
ex 2: how to calculate the percent composition if given the masses of each component?
1. find the molar mass of all the elements in the compound in g/mol
2. find the molecular mass of the entire compound
3. divide the components molar mass by the entire molecule mass
4. multiply it by 100% to get percent composition
ex 2: what would happen if you didn't dissolve the panacetin in the DCM well enough?
not all the aspirin would be separated from the panacetin and it would not fully precipitate
ex 2: what would happen if you didn't mix both layers well enough during separation?
they would not be fully separated, and the aspirin would not be fully removed from the DCM layer
ex 2: what would happen if you mixed them too vigorously and created an emulsion?
an unwanted solid would be formed
ex 2: what would happen if you didn't use enough sodium hydroxide to separate?
not all of the aspirin would be converted to the salt
ex 2: what would happen if you didn't use enough HCl in the sodium acetylsalicylate solution?
not all of the salt would be protonated and it would be converted back to aspirin
ex 2: what would happen if you confused the beakers? what would be left behind after the distillation? what would happen when you add the HCl?
1) DCM would be neutral
2) unknown will not be left behind
3) HCl would protonate one of them
ex 3 summary
- we recrystallize by boiling the solution containing our unknown to remove impurities
- what makes a good solvent? doesn't react with unknown, boils below compounds melting point, dissolves our unknown at high temperatures but not at cold temperatures
ex 3: melting point determination
melting point analysis determines identity and purity.
- only works for solid compounds which have known melting points
- reported as a range
- the closer the melting points is to the lit value, the more likely it is that compound
the presence of impurities do 2 things:
1. depresses melting point
2. broadens the range
ex 3: percent recovery formula
amount after recrystallization/amount before recrystallization x 100 = % recovery
ex 3: what would we do if there is undissolved solid in the boiling water?
hot filtration
ex 3: why does melting point work in this experiment and why don't we use it in other experiments?
melting point works for this experiment because the product is a solid, and the melting point of the solid is known. this doesn't work for other experiments because not all products are solid and we don't know the melting points for all products
ex 3: what would happen if there was residual aspirin in the unknown?
the melting point would be inaccurate and the purity would be decreased
ex 3: what would happen if enough water was used for phenacetin?
too much water could dissolve the phenacetin in the mixture
ex 3: what would happen if the unknown wasn't dry during melting point analysis?
there would be impurities in the mixture and the melting point would be inaccurate
IR: what units do we use for IR and why?
wavelengths (cm^-1) are used because they are directly proportional to energy
IR: how could IR be used to distinguish between these two compounds: CH3CH2CH2OH and CH3CH2OCH3?
there would be an OH group peak on one IR, but not on the other
IR: how could IR be used to distinguish between these two compounds: C6H10CN vs C6H10CH2NH3?
there would be an NH3 peak on one, and a CN peak on the other
IR: which compound would exhibit intense IR absorption at 1750 cm^-1?
a. C5H10O
b. C5H8CH2Oh
c. CH3COC6H10
c. CH3COC6H10
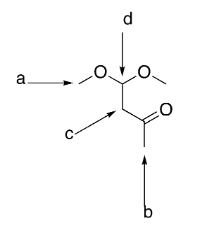
which group is the most deshielded?
d
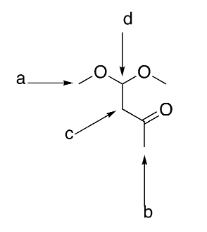
which group has the most intense integration?
a
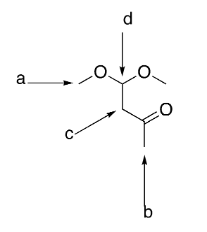
which group(s) exhibit singlet splitting?
a and b
how many signals would be exhibited by the structure?
4
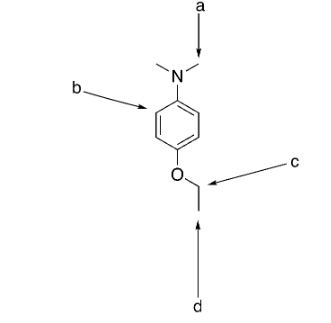
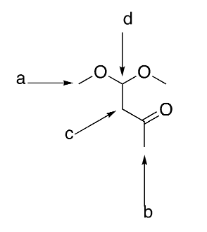
which group is the most deshielded?
b
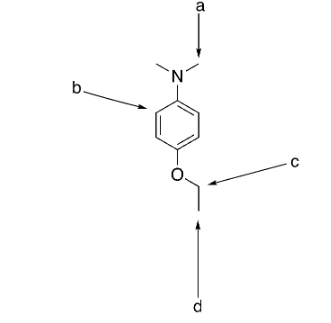
which group has the most intense integration?
a
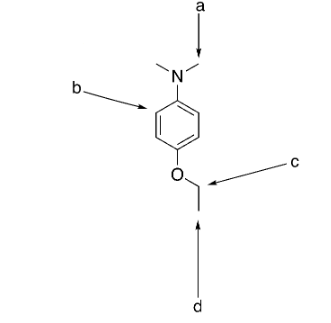
which group(s) exhibit singlet splitting?
a and b
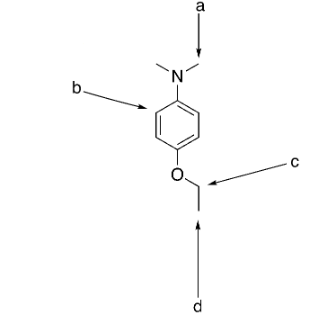
how many signals would be exhibited by the structure to the right?
4
gas chromatography
separates compounds based on boiling point and polarity
- separation is generated by the opposition of forces between mobile phase and stationary phase
- normal phase: polar stationary phase and nonpolar mobile phase
- reverse phase: nonpolar stationary phase and polar mobile phase
ex 5 procedure
1. in a round bottom flask, place 150 mmol of isopentyl alcohol in a flask with excess acetic acid and aqueous sulfuric acid
2. reflux making sure not to cap the condenser
3. collect the organic layer using a separatory funnel with a water wash first
4. wash with sodium bicarbonate to remove all residual acid
5. distill the product
6. analyze using IR, NMR, and GC
ex 5: why do we mix isopentyl acid with excess acetic acid?
excess acetic acid drives the reaction to the right
ex 5: why do we mix isopentyl acid with sulfuric acid?
sulfuric acid provides the acid catalyst
ex 5: when refluxing, why do we not cap the condenser?
capping will create a pressurized bomb
ex 5: when separating, why do we wash with water first?
washing with sodium bicarbonate first will cause a violent reaction
ex 5: why do we distill the product?
isopentyl alcohol and isopentyl acetate are both miscible with each other
ex 5 key concepts/theories
- refluxing reactions helps in overcoming activation energy for otherwise slow processes
- since it is a reversible reaction, it makes it challenging to have 100% yield
- we need to use liquid-liquid distillation to separate the isopentyl alcohol from the isopentyl acetate
ex 5: why can't we just do a liquid-liquid separation in the separatory funnel?
the reaction needs to be heated in order to speed up the reaction
ex 5: what would happen if the catalyst wasn't added?
the reaction would take longer to react
ex 5: what would happen if the water wasn't running in the condenser?
the vapor would not be cooled and the product would escape out of the condenser
ex 5: what would happen if excess water was in the round bottom flask during distillation?
the water would distill out first
ex 5: what would happen if you omitted the sodium bicarbonate wash?
the mixture would have impurities, not all of the acid would be removed
ex 5: what would happen if you refluxed for 2 hours instead of 1?
the mixture would revert back to its starting material
ex 15 summary
- thin layer chromatography analyzes compounds and separates based on the distance travelled up the plate
- only able to analyze compounds which can be seen with I2 or UV
ex 15: in normal phase TLC, which compounds have the largest Rf?
the least polar compounds travel the farthest and thus have the largest Rf
ex 15: what would happen if the solvent wick wasn't used or the foil wasn't put on top of the beaker?
- if the solvent wick wasn't used, the gas from solvent would not be distributed evenly, and there would be streaks on the TLC plate
- if the foil wasn't put on top of the beaker, the solvent would eventually evaporate
ex 15: what would happen if the spotting line was drawn too hard?
the components wouldn't move up the plate
ex 15: what would happen if the solvent line was above the spotting line?
the TLC would not run
ex 4 procedure
1. add wintergreen oil to aqueous sodium hydroxide and reflux for 30 mins or until solution is clear and absent of any layers
2. precipitate out solution using excess sulfuric acid
3. collect product via vacuum filtration and recrystallize based on the boiling water solubility of salicylic acid
4. collect product again via vacuum filtration
5. analyze using melting point determination (pure product and 1:1 mixture with benzene synthesized salicylic acid)
ex 4: why do we reflux solution until it is clear and absent of any layers
absence of layers indicates there is no remaining methyl salicylate
ex 4: why do we precipitate out solution using excess sulfuric acid?
neutralize the extra NaOH in the reaction flask
ex 4: why do we analyze pure product and 1:1 mixture with benzene synthesized salicylic acid?
to determine if synthesizing SA from benzene creates a less pure product
ex 4 key concepts/theories
NaOH is both a nucleophile and base in this reaction mechanism which is why it is important to use at least 2 equivalents of NaOH
ex 4: what makes a good solvent?
1. doesn't react with the reactants
2. dissolves the reactants
3. boils below the compound's melting point yet has a high enough BP to provide enough activation energy
ex 4: what would happen if an insufficient amount of NaOH was used?
the methyl salicylate would be protonated, and the yield would be decreased
ex 4: what would happen if DCM was used as the solvent?
1. the reaction would not be able to overcome activation energy
2. the reaction would be very flammable
ex 4: what would happen if HCl was used to precipitate the salicylic acid out of the solution instead of H2SO4?
no effect