PCR as a Diagnostic Tool (Week 1, Mod 9)
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
14 Terms
What is the Polymerase Chain Reaction? (PCR)
The amplification of a specific DNA sequence (target) within a sample
Is used to detect / isolate specific nucleic acid sequences related to particular conditions or diseases (viral, bacterial, autoimmune, etc.)
What are the 5 key components that are needed to complete a PCR test?
Template DNA – e.g. blood, tissue, swabs
Primers – short synthetic oligonucleotides; starting points for DNA synthesis, defining the region of the template DNA to be copied
Taq Polymerase – DNA polymerase
Deoxynucleotide triphosphates (dNTPs) – DNA building blocks
Buffer (Mg²⁺)
What are the 3 major steps of PCR?
1) DENATURING - Target DNA is denatured into separate strands (95 degrees C)
2) ANNEALING - Reaction is COOLED to allow primers to anneal to the target DNA (55-65 degrees C)
3) EXTENDING - Primers are then extended using Taq polymerase (72 degrees C)
Cycle is then repeated 25-30 times, resulting in billions of copies (amplicons)
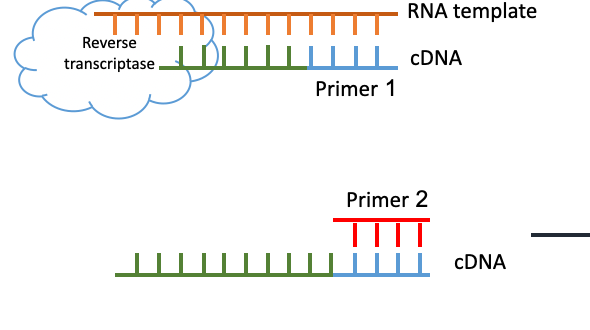
What is the major difference between PCR and Reverse Transcriptase PCR (RT-PCR)?
Starting out with an RNA segment instead of DNA… need to first convert it to a complementary DNA (cDNA) using the reverse transcriptase enzyme
After that is done, the cDNA is then used in the classic PCR reaction
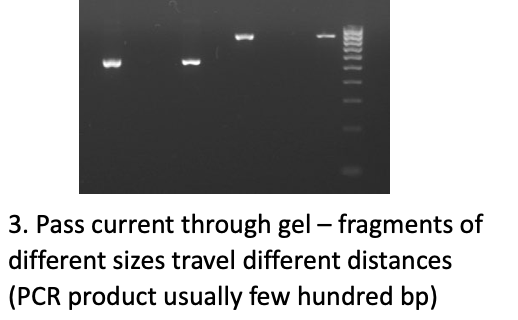
What is the difference between Conventional PCR and qPCR (aka Real TIme PCR)?
Conventional PCR → gives you QUALITATIVE RESULTS, not quantitative like qPCR
Product goes through electrophoresis gel; if something is highlighted, that means that your target DNA WAS in the sample
BUT doesn’t say how much of your target was in the sample either
Usually have to recover the product for further investigation via gel / capillary electrophoresis
SEE IMAGE: this would be the last step in Conventional PCR; shows the different size of your products as it travels down the gel in comparison to the marker
What is Real-time PCR (qPCR)?
Is QUANTITATIVE
Based on PCR principles
.. product is measured as the reaction progresses, in real time, with product quantification after each cycle.
.. To enable detection, the amplified product is labelled with a fluorescent dye
What are the 2 different fluorescent dyes used in qPCR? Which one is better to use?
SYBR Green
non-sequence-specific fluorescent dyes that intercalates with all dsDNA product
Fluorescence proportional to all amplified product (specific and non-specific)
TaqMan
sequence-specific DNA probes attached to fluorochrome are added which bind through sequence complementarity to dsDNA product
fluorescence proportional to specific product
TaqMan would be best to use… more specific
What are the 2 different phases in qPCR data as its being recorded?
1) Exponential phase
Reagents are abundant and non-limiting
At 100% efficiency doubling of product each cycle
2) Non-exponential phase
Reagents running out until they are exhausted
No further amplification can occur
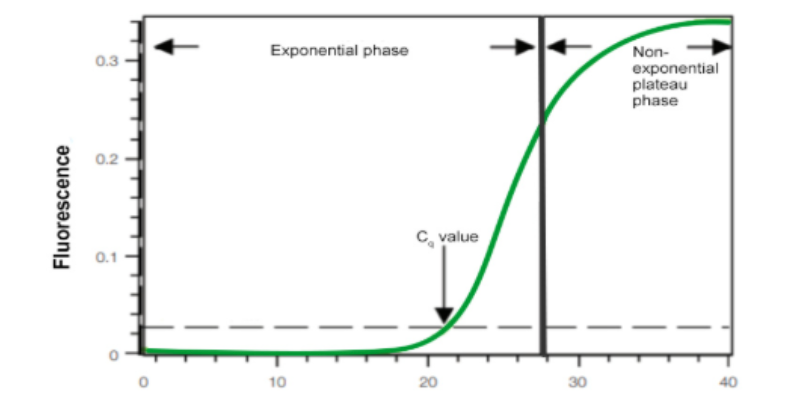
What is the threshold line in this graph? What does the Ct value represent?
Threshold line – machine calculated level of fluorescence which is (statistically)
significantly higher than baseline
Ct (threshold cycle) – cycle number at which sample fluorescence crosses threshold; tells you how much target was in your sample
Low Ct = high amount of target in samples, high Ct = low amount of target in samples
What are 4 things that could affect the PCR results?
Poor sample collection technique
Sample contamination
Sample degradation: RNA viruses (RNA degrades faster than DNA)
PCR inhibitory substances - Substances contained within the clinical sample that interfere with the PCR reaction: FALSE NEGATIVE
E.g. (Lithium) heparin, fluorescein, cat litter, charcoal, Heme, bilirubin, bile salts (faeces).
What are 2 things that could cause a false positive PCR?
Laboratory contamination
Non-specific primers: then some products may not be of desired sequence
What are 3 things that could cause a false negative PCR?
Non-specific primers: target sequence may not be amplified efficiently
Primers may not bind to mutant strains
Reaction failed
What are 7 advantages to using PCR testing?
Rapid (24-48hr report), sensitive, specific diagnosis
Identify pathogens non-cultivable/slow to culture/dangerous to culture/overgrown by other agents
Can be performed on formalin-fixed, paraffin-embedded tissues
Identification of carriers + shedders
Strain-specific identification
Vaccine vs field infections (sequence differences)
Quantitative: pathogen load
What are 3 limitations in PCR?
No information on viability/infectivity
No data on antimicrobial susceptibility (bacterial pathogens)
Commensal or not clinically significant