Electrophilic Addition: Mechanism and Regioselectivity
1/19
Earn XP
Description and Tags
LGs 2.6 & 2.7
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
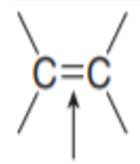
What kind of bond is the arrow pointing at?
Double Bond
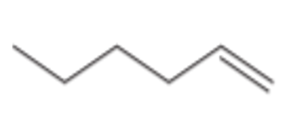
What type of alkene is this?
Terminal Alkene
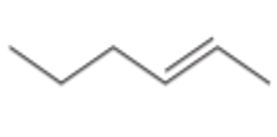
What type of alkene is this?
Internal Alkene
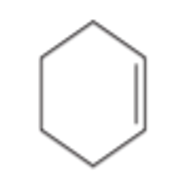
What type of alkene is this?
Cycloalkene
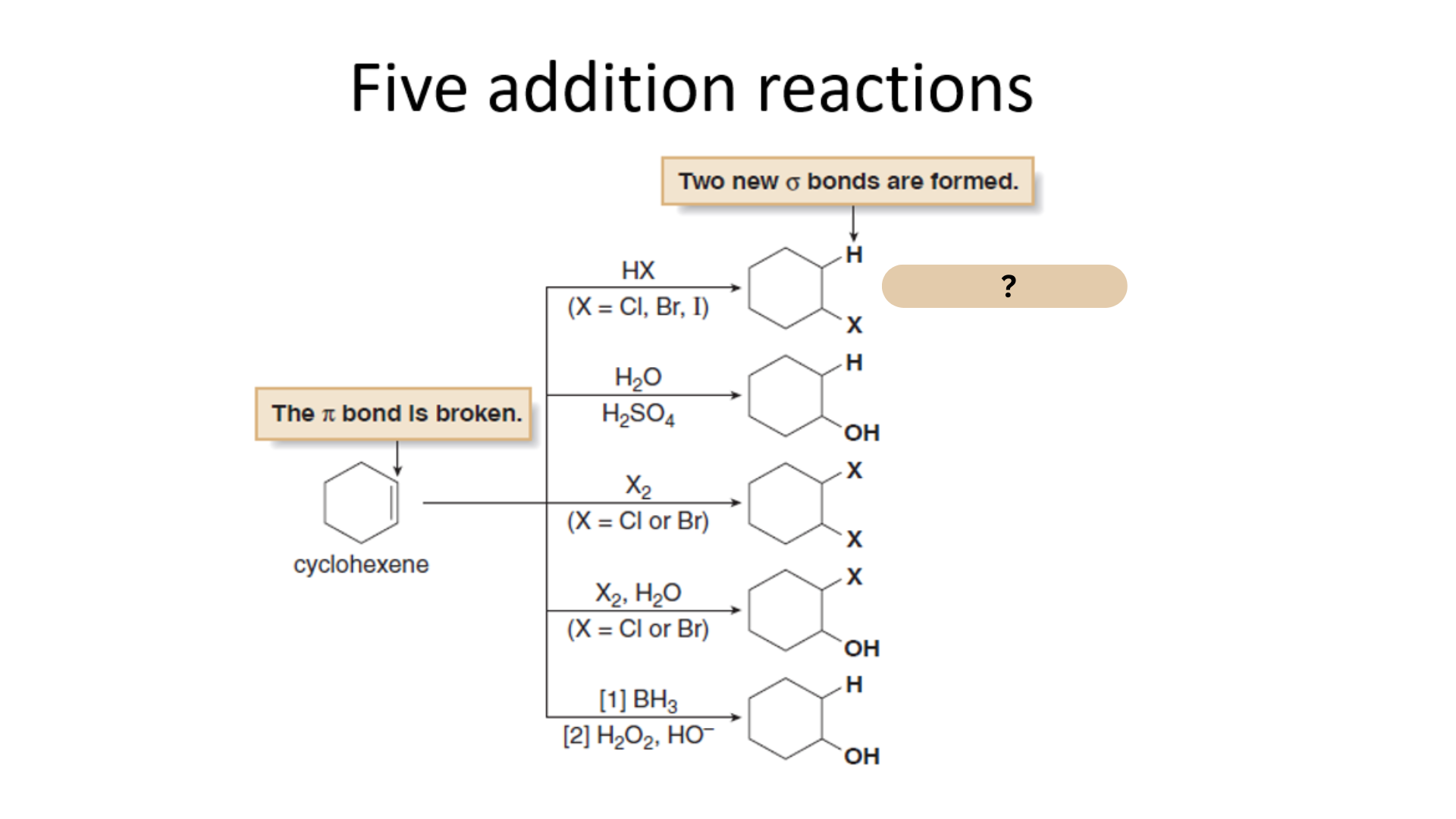
Among the Five Electrophilic Addition Reactions, what is this reaction?
Hydrohalogenation
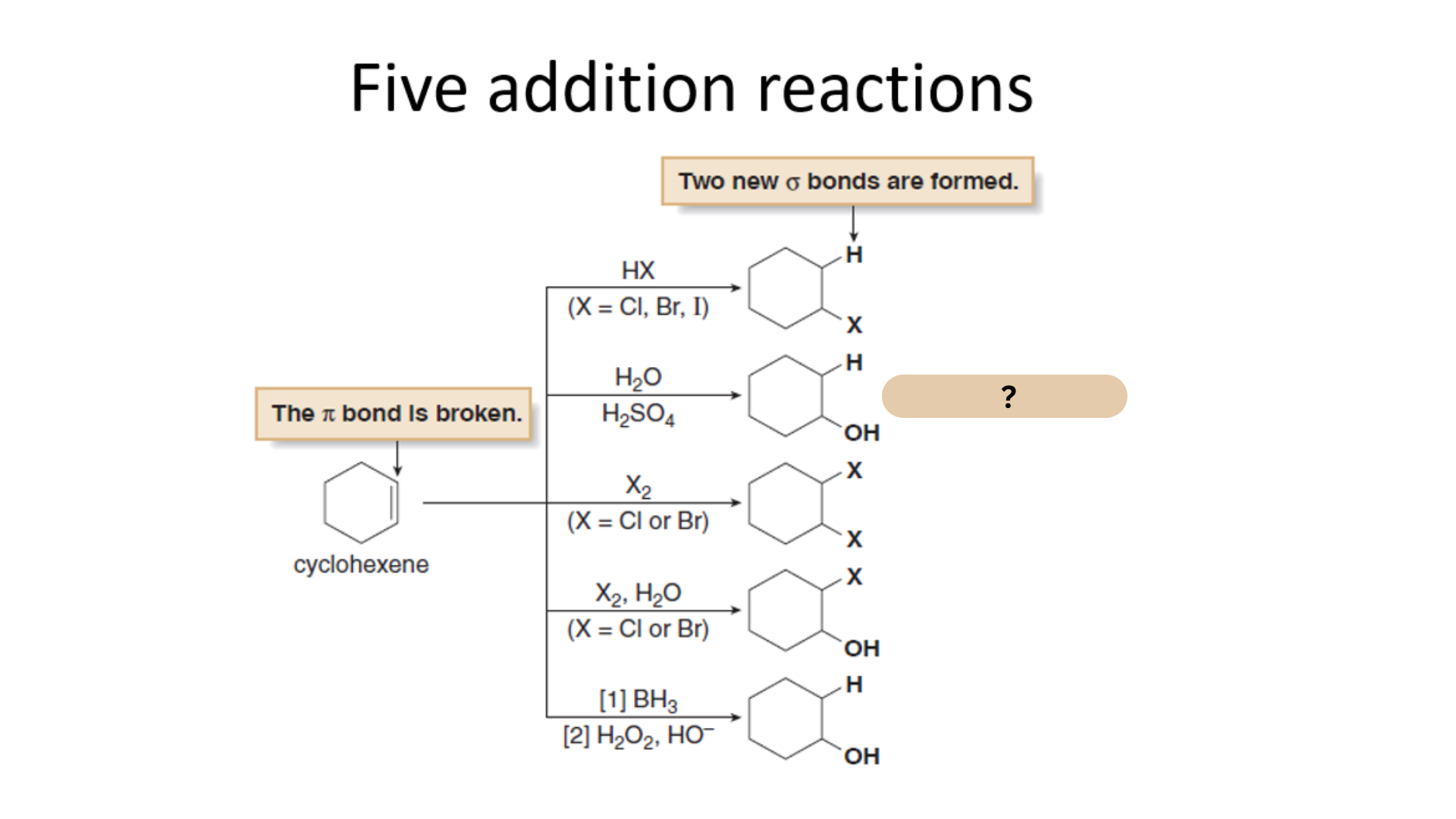
Among the Five Electrophilic Addition Reactions, what is this reaction?
Hydration
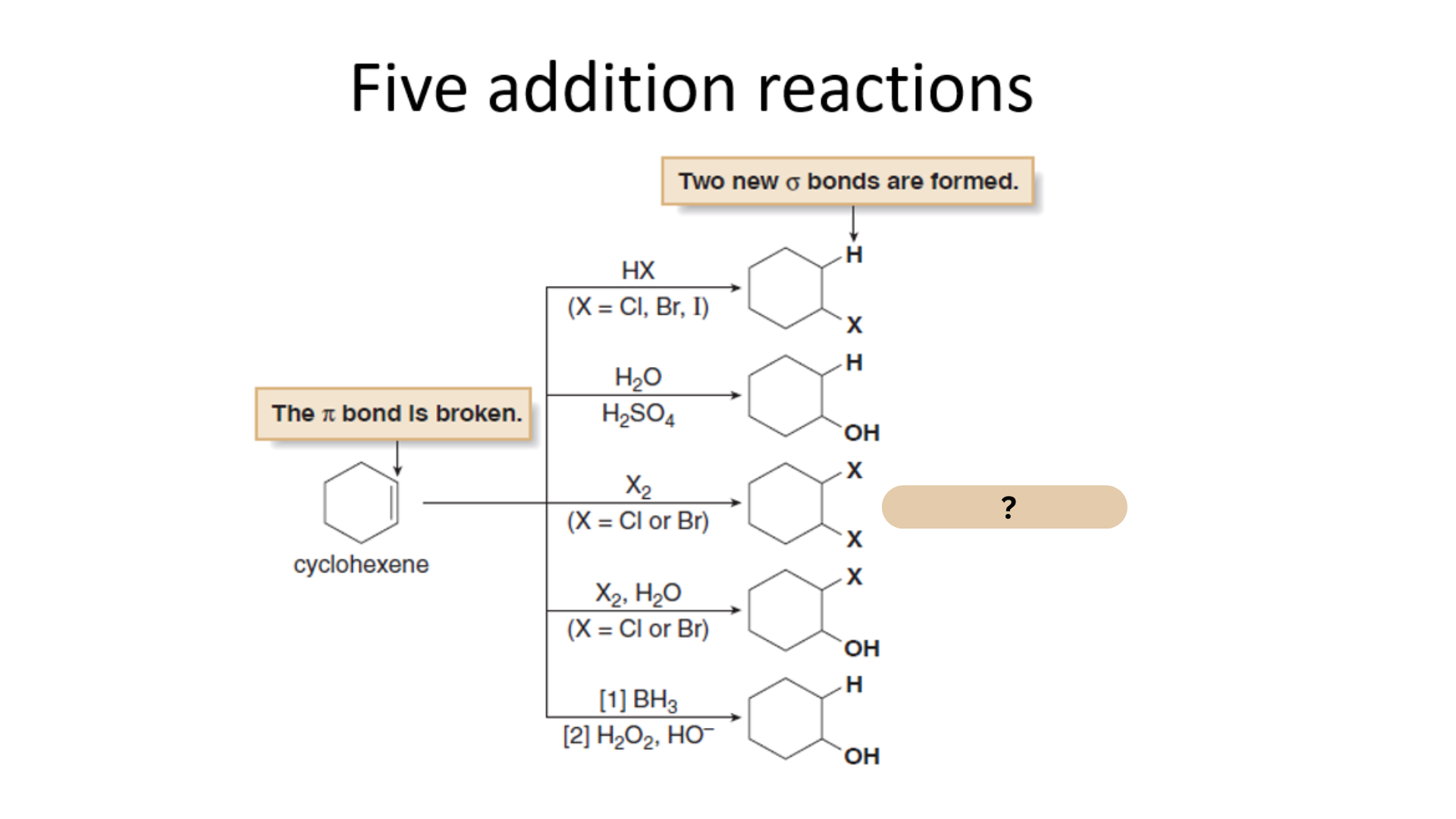
Among the Five Electrophilic Addition Reactions, what is this reaction?
Halogenation
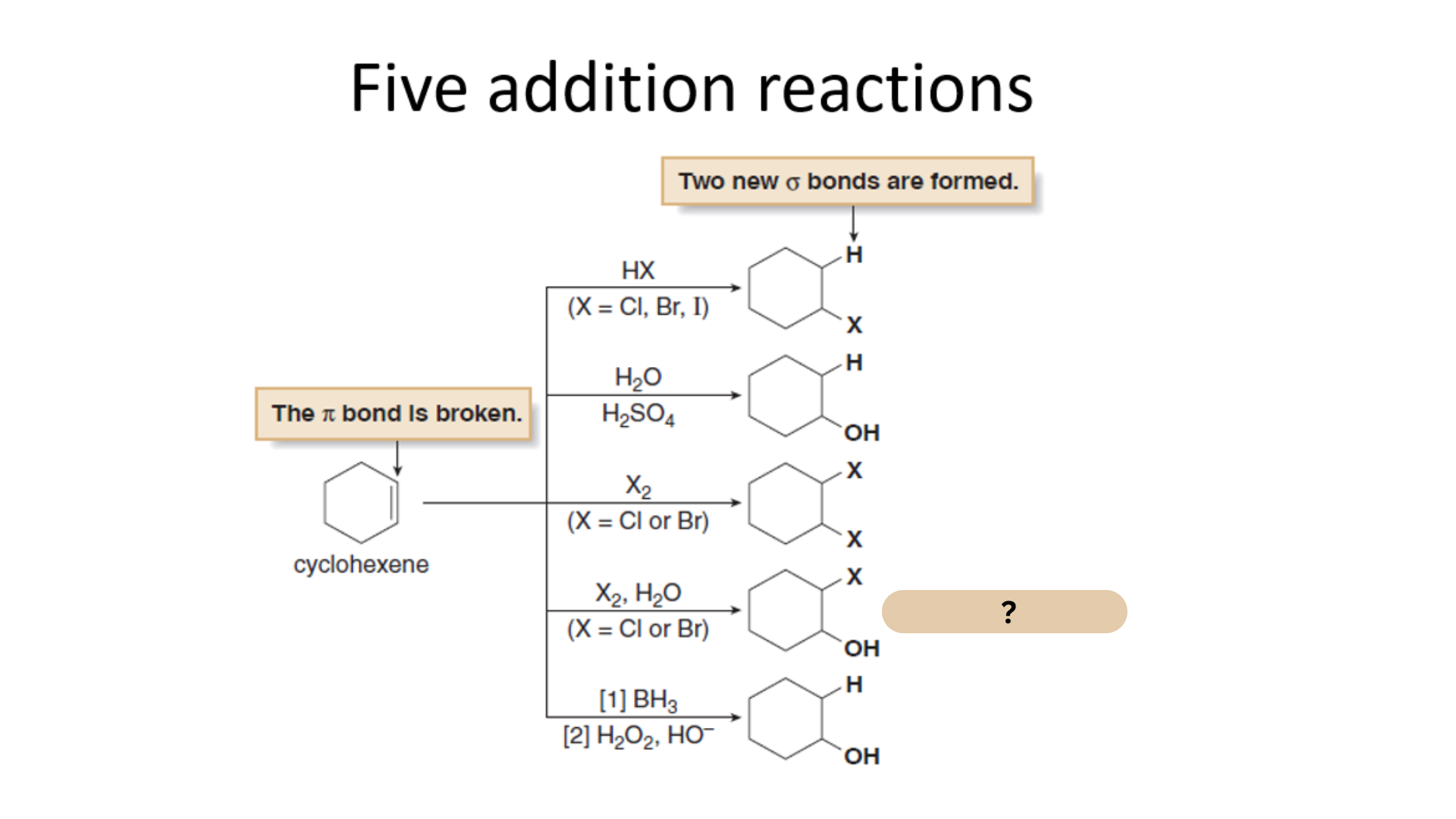
Among the Five Electrophilic Addition Reactions, what is this reaction?
Halohydrin Formation
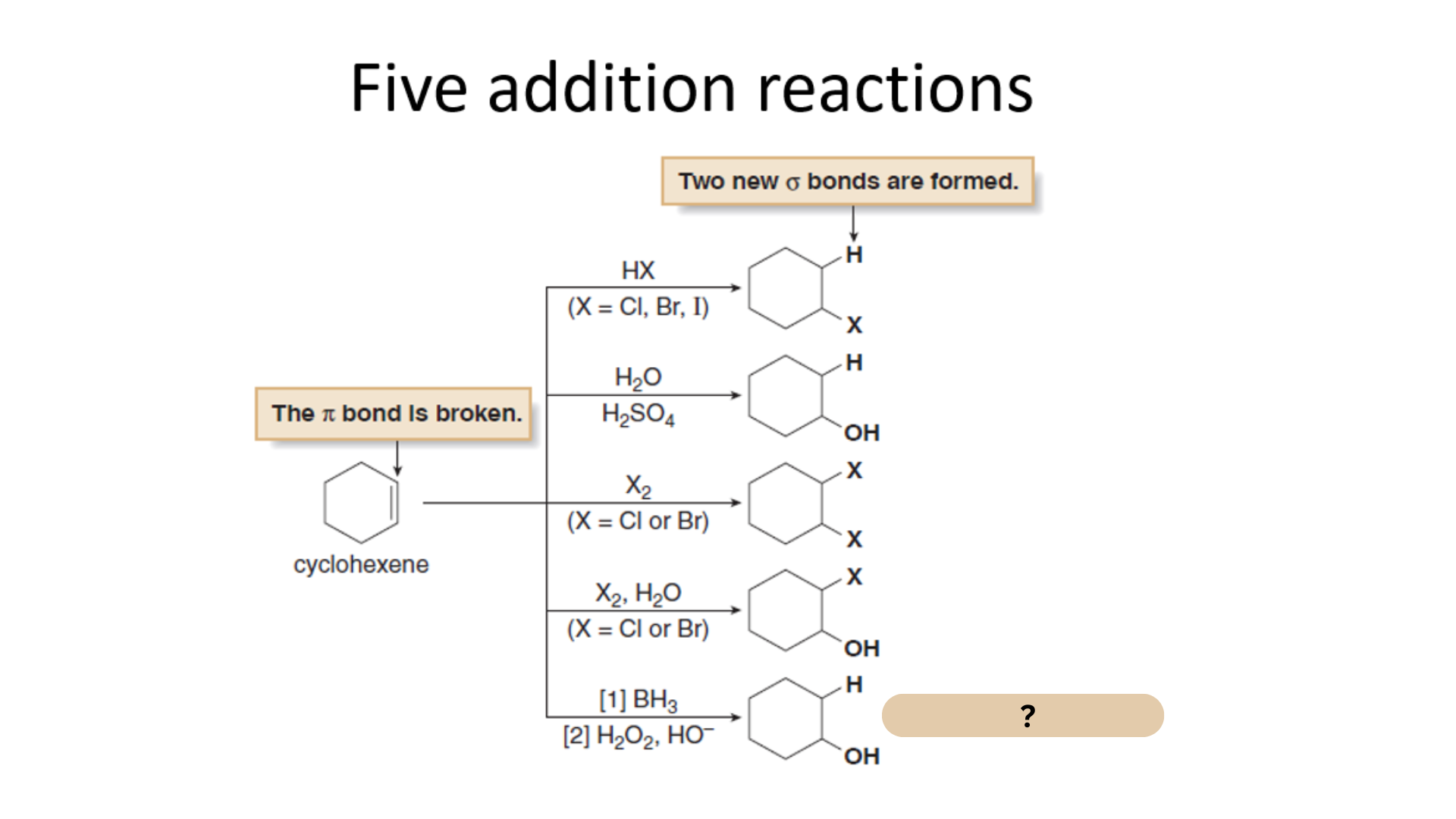
Among the Five Electrophilic Addition Reactions, what is this reaction?
Hydroboration-oxidation
In organic compounds, this is a polar reaction characterized by the incorporation of an electrophile to a carbon-carbon double bond to form a saturated product.
What does this description refer to?
Electrophilic Addition
•Every reaction of alkenes involves addition: the л bond is always broken.
•Because alkenes are electron rich, simple alkenes do not react with nucleophiles or bases, reagents that are themselves electron rich. Alkenes react with electrophiles.
Which among the Five Electrophilic Addition Reactions refers to the addition of hydrogen halides HX (X = Cl, Br, and I) to alkenes to form alkyl halides.
Hydrohalogenation
Which among the Five Electrophilic Addition Reactions refers to the Electrophilic Addition of HX?
Hydrohalogenation
Which among the Five Electrophilic Addition Reactions refers to the Electrophilic Addition of Water (H2O)?
Hydration
Which among the Five Electrophilic Addition Reactions refers to the addition of water to an alkene to form an alcohol. H2O itself is too weak an acid to protonate an alkene, but with added H2SO4, H3O+ is formed and addition readily occurs.
Hydration
Which among the Five Electrophilic Addition Reactions refers to the addition of halogen X2 (X = Cl or Br) to an alkene, forming a vicinal dihalide.
Halogenation
Which among the Five Electrophilic Addition Reactions refers to the treatment of an alkene with a halogen X2 and H2O forms a halohydrin by addition of the elements of X and OH to the double bond.
Halohydrin Formation
Which among the Five Electrophilic Addition Reactions converts an alkene to an alkylborane then into an alcohol
Hydroboration-oxibation
___________ occurs in chemical reactions where one reaction site is preferred over another?
Regioselectivity