NCEA Level 3 Chemistry - Thermochemical properties
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
Define Covalent bonding
the strong attraction between two positive nuclei and one or more shared pairs of electrons
What is a polar substance
substance with distinct regions of positive and negative charge
What are the exceptions to the Octet rule
Be - only 2 bonds wo/ lone pairs
B, Al - only 3 bonds wo/ lone pairs
H - only 1 bond wo/ lone pairs
180° bond angle name
linear
120° bond angle name
trigonal planar
109.5° bond angle name
tetrahedral
What bond angle does molecules with 2 electron areas have
180°
What bond angle does molecules with 3 electron areas have
120°
What bond angle does molecules with 4 electron areas have
109.5°
2 bonding areas 0 non-bonding
linear shape
3 bonding areas 0 non-bonding
trigonal planar
2 bonding areas 1 non-bonding
bent (120°)
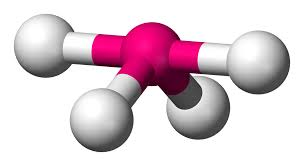
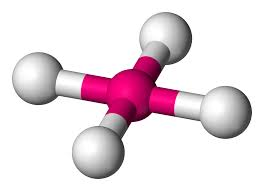
4 bonding areas 0 non-bonding
tetrahedral
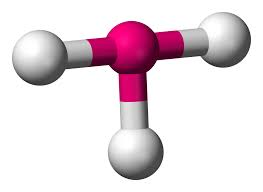
3 bonding areas 1 non-bonding
trigonal pyramid
2 bonding areas 2 non-bonding
bent (109.5°)
What does bond polarity require
the atoms in the bond
Define electronegativity
the ability of an atom to attract the bonding pair of electrons in a covalent bond. - closer to Fluorine=more electronegativity
What determines an asymmetrical molecule
lone pairs around the central atom
Define enthalpy
a thermodynamic quantity equivalent to the total heat content of a system
Enthalpy of breaking bonds
Endothermic-requires energy
Enthalpy of forming bonds
Exothermic-releases energy
Define H (kJ)
Amount of energy/heat released or absorbed by the system
Define ∆ᵣH⁰ (kJmol⁻¹)
Change in the enthalpy of a reaction system
What happens in an endothermic reaction
temp of surroundings decreases
bonds are broken
∆ᵣH⁰ is POSITIVE
The products have more enthalpy than reactants
What happens in an exothermic reaction
temp of surroundings increases
bonds are formed
∆ᵣH⁰ is NEGATIVE
The products have less enthalpy than reactants
VSEPR theory
angles are controlled by the total # of electron areas around the central atom
shape is controlled by the number of bonding and non-bonding electron areas around the central atom
Shape code
The shape and angle are controlled by minimising the repulsion of the electron areas around the central ______ atom
The electron arrangement that minimises repulsion for __electron areas is ____ which results in an angle of____ Because ____electron areas are bonding and___ non-bonding, it results in a ____ shape with a bond angle of _____.
Polarity Code
Molecular polarity is controlled by the distribution of charge about the central ____atom.
The __-__ bonds are polar because__are more electronegative than __.
This results in uneven sharing of electrons which forms bond dipoles.
This molecule has a____shape, which is symmetric/asymmetric, as the bond dipoles are all the same/different they will cancel/not cancel and the molecule will be non-polar/polar.
∆ᵣH⁰=
bonds broken - bonds formed
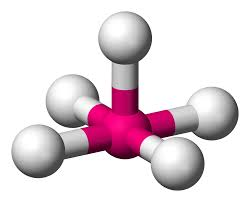
5 bonding areas 0 non-bonding
Trigonal bipyramid
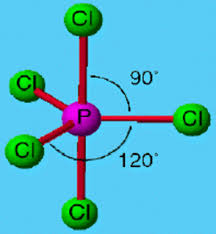
4 bonding 1 non-bonding
seesaw
3 bonding 2 non-bonding area
T shape
2 bonding 3 non-bonding area
Linear
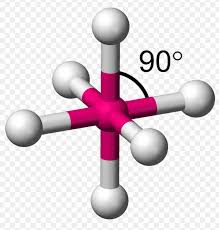
6 bonding 0 non-bonding areas
octohedral
5 bonding 1 non-bonding area
square based pyramid
4 bonding 2 non bonding areas
square planar
what are the 3 factors affecting electrostatic attraction between nucleus and valence electron
Nuclear charge (number of proton) greater the charge=greater attraction to valence electron
Number of energy levels/distance of electrons
Higher energy levels are further away from the nucleus
valence electrons are shielded more from the nucleus
More or less valence electron-electron repulsion
Trends down a group paragraph
Going down a group. the valence electrons are in energy levels further from the nucleus with increase shielding from the inner shells. Although the number of protons increase down a group , this attraction is offset by the increase in distance between the nucleus and the valence elections so, the electrostatic attraction between the positive nucleus and its vanlece electrons decrease.
Trends across a period paragraph
Across a period, the valence electrons are added to the same energy levels with the same shielding from the inner levels. The number of protons increase from period to period, This results in an increased electrostatic attraction between the positive nucleus and the valence electrons across a period.
Atomic radius trend
decreases across a period
increases doen a group
Ionisation energy meaning
The energy required to remove one mole of electrons froom one mole of gaseous positive ions
e.g Na₍₉₎→Na⁺₍₉₎+e⁻ remeber state symbol
Ionisation energy trend
Increases across a period
Decreases down a group
Electronegativity trend
Increases across a period
decreases down a group
Explain why anions are larger than the atom
When an anion forms, the atom gains electrons in the valence shell, while the nuclear charge remains constant, This results in greater valence electron - electron repulsion, resulting in the anion having greater radius than atoms
Explain why cations are smaller than the atom
When an atom becomes a cation, an electron/s are removed from the valence shell. This reduces electron - electron repulsion or removes an entire energy level.
Types of intermolecular forces
Temporary dipole - induced dipole
Permanent dipole - dipole forces
Hydrogen bonding
Temporary dipole - induced dipole
Weakest of all intermolecular forces
Temporary charge imbalances caused by random movement of electrons inducing a charge in an adjacent molecule
As #e-/molar mass increase b.p increases
The greater the S.A, the greater the td-id forces
Permanent dipole - dipole forces
Occurs between polar molecules
acts in addition to td-id
Hydrogen bonding
strongest intermolecular force
requires H bonded to a highly electronegative element (N, O, F)
AND a non bonding electron pair on (N, O, F) of an adjacent molecule
What is enthalpy of combustion ∆cH°
Enthalpy change when one mole of the substance is completely burnt under standard conditions e.g ∆cH°(H₂(g)) is H₂(g)+1/2 O₂(g)→H₂O(l)
What is enthalpy for formation ∆fH°
enthalpy change to form 1 mole from its elements, all in their standard state e.g.
∆fH° (CO₂(g)) is C(s)+O₂(g)→CO₂(g)
What is enthalpy of vaporisation ∆vapH°
enthalpy change when one mole of the substance changes from a liquid to gas eg
∆vapH°(H₂0(l)) H₂0(l)→H₂0(g)
what is enthalpy of fusion ∆fusH°
enthalpy change when one mole of the substance changes from a solid to a liquid
∆fusH°(C₅H₁₀(s)) C₅H₁₀(s)→C₅H₁₀(l)
what is enthalpy of sublimation ∆subH°
enthalpy change when one mole of substance changes from a solid to a gas
∆subH°(CO₂(s)) CO₂(s)→CO₂(g)