Chemistry I Lesson 10: Titrations Part II
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
What is the pH of a 143.8 mL solution of .32 M NH3 (pKb = 4.75) when you add 45.9 mL of .13 M HCl?
(A) 5.61
(B) 6.98
(C) 8.32
(D) 10.43
(D) 10.43
45.9 mL HCl ⋅ 1 L / 1000 mL ⋅ .13 moles HCl / 1 L HCl = approx. 5 ⋅ 10^-3 moles HCl (actual: 5.967 ⋅ 10^-3)
143.8 mL NH3 ⋅ 1 L / 1000 mL ⋅ .32 moles NH3 / 1 L NH3 = approx. 45 ⋅ 10^-3 (actual: 46.016 ⋅ 10^-3)
45 ⋅ 10^-3 - 5 ⋅ 10^-3 = 40 ⋅ 10^-3 moles NH3
5 ⋅ 10^-3 moles NH4+
Once again, you can use the Henderson-Hasselbach equation here, but it will slow you down big time. Simply notice that the concentration of base (NH3) will be about 10x greater than the conjugate acid (NH4+), making the solution approximately 1 pH unit higher than the pKa of NH3.
Also notice that the pKb was given, not pKa (pKb = pKw - pKa = 14 - 4.75 = 9.25)
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
When will a pH indicator change color?
In a specific pH range for that indicator.
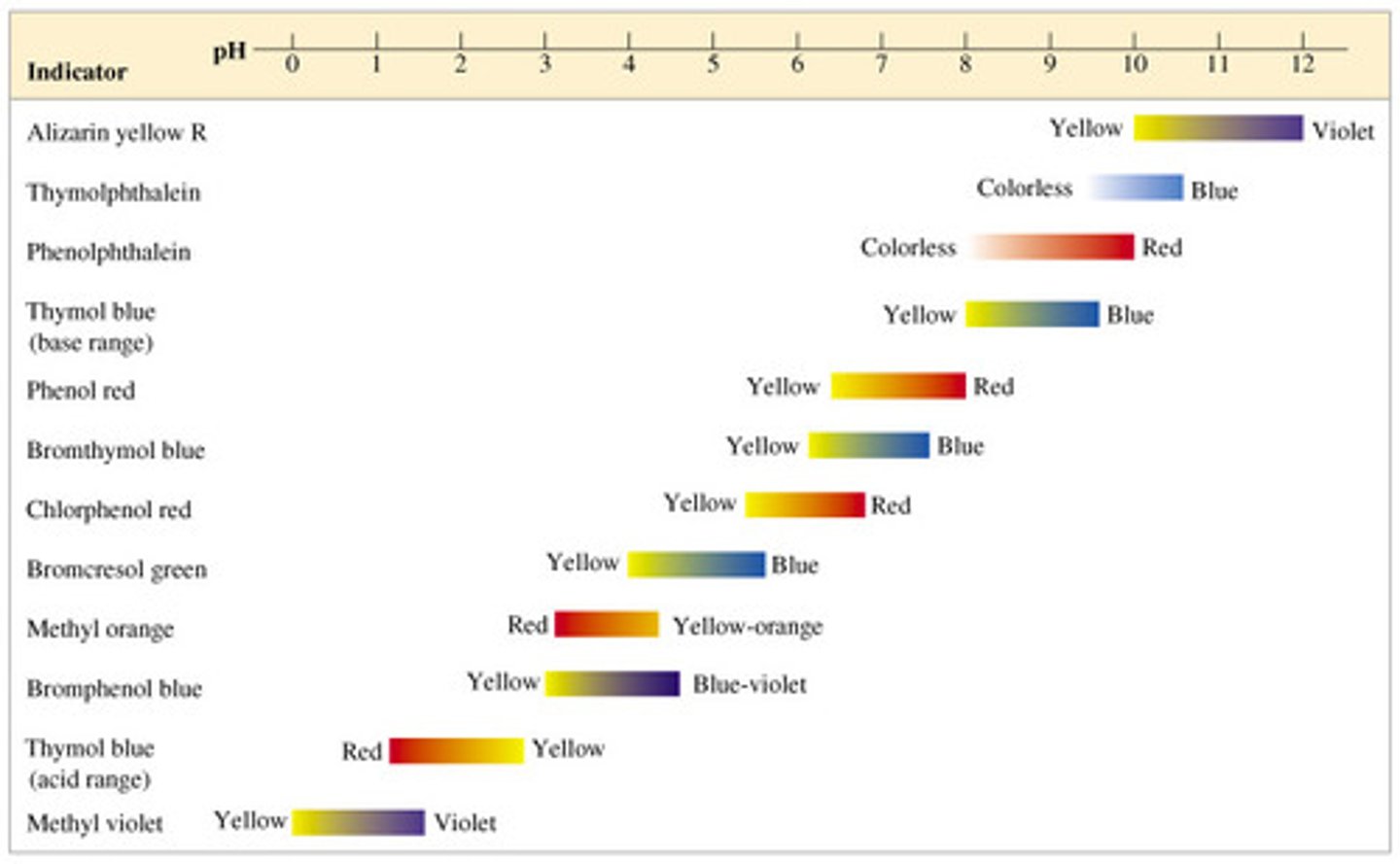
The pH ranges for methyl red, bromthymol blue, and phenolphthalein are 4.4 - 6.2, 6.0 - 7.6, and 8.2 - 10, respectively. Which would best be used for titrating a strong acid with a strong base in which the steep part of the titration curve ranges from a pH of 1.8 to 12.7?
I. Methyl red
II. Bromthymol blue
III. Phenolphthalein
(A) I Only
(B) II Only
(C) III Only
(D) I, II, and III
(D) I, II, and III
Because the curve is so steep, any one of these indicators would change color near the equivalence point.
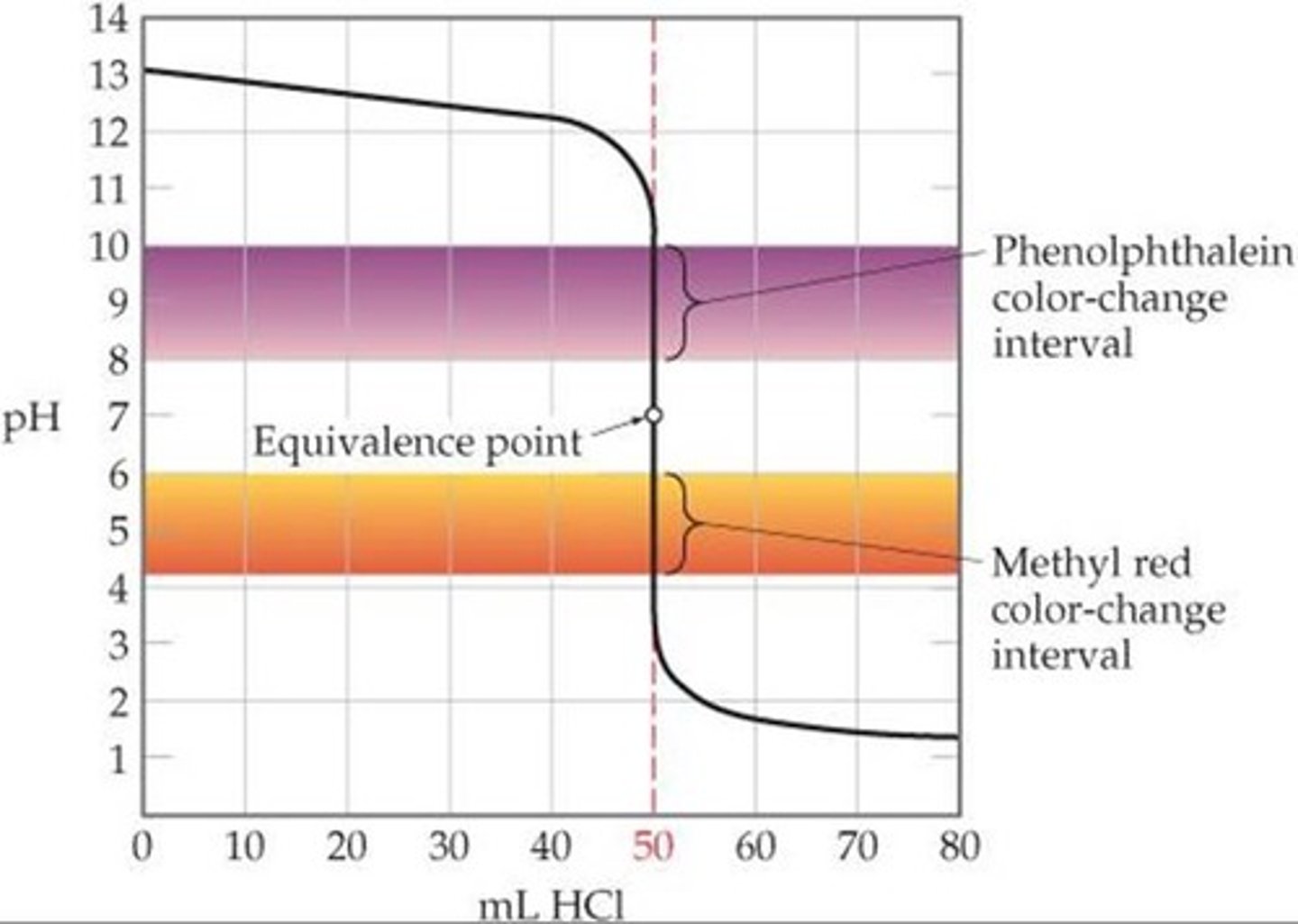
The pH ranges for methyl red, bromthymol blue, and phenolphthalein are 4.4 - 6.2, 6.0 - 7.6, and 8.2 - 10, respectively. Which would best be used for titrating a weak acid with a strong base in which the steep part of the titration curve ranges from a pH of 6.2 to 11.9?
I. Methyl red
II. Bromthymol blue
III. Phenolphthalein
(A) I Only
(B) II Only
(C) III Only
(D) I, II, and III
(C) III Only
Methyl red and bromthymol blue would change colors before the steep curve, giving you an inaccurate measurement of how much base is added to reach the equivalence point.
The pH ranges for methyl red, bromthymol blue, and phenolphthalein are 4.4 - 6.2, 6.0 - 7.6, and 8.2 - 10, respectively. Which would best be used for titrating a weak base with a strong acid in which the steep part of the titration curve ranges from a pH of 7.4 to 3.5?
I. Methyl red
II. Bromthymol blue
III. Phenolphthalein
(A) I Only
(B) II Only
(C) III Only
(D) I, II, and III
(A) I Only
Bromthymol blue and phenolphthalein would change colors before you reach the steep part of the curve.
CRB Fill in the blank: For a weak acid titrated with a strong base, the equivalence point will occur at ________.
(A) pH > 7
(B) pH = 7
(C) pH < 7
(D) Not enough information to determine
(A) pH > 7
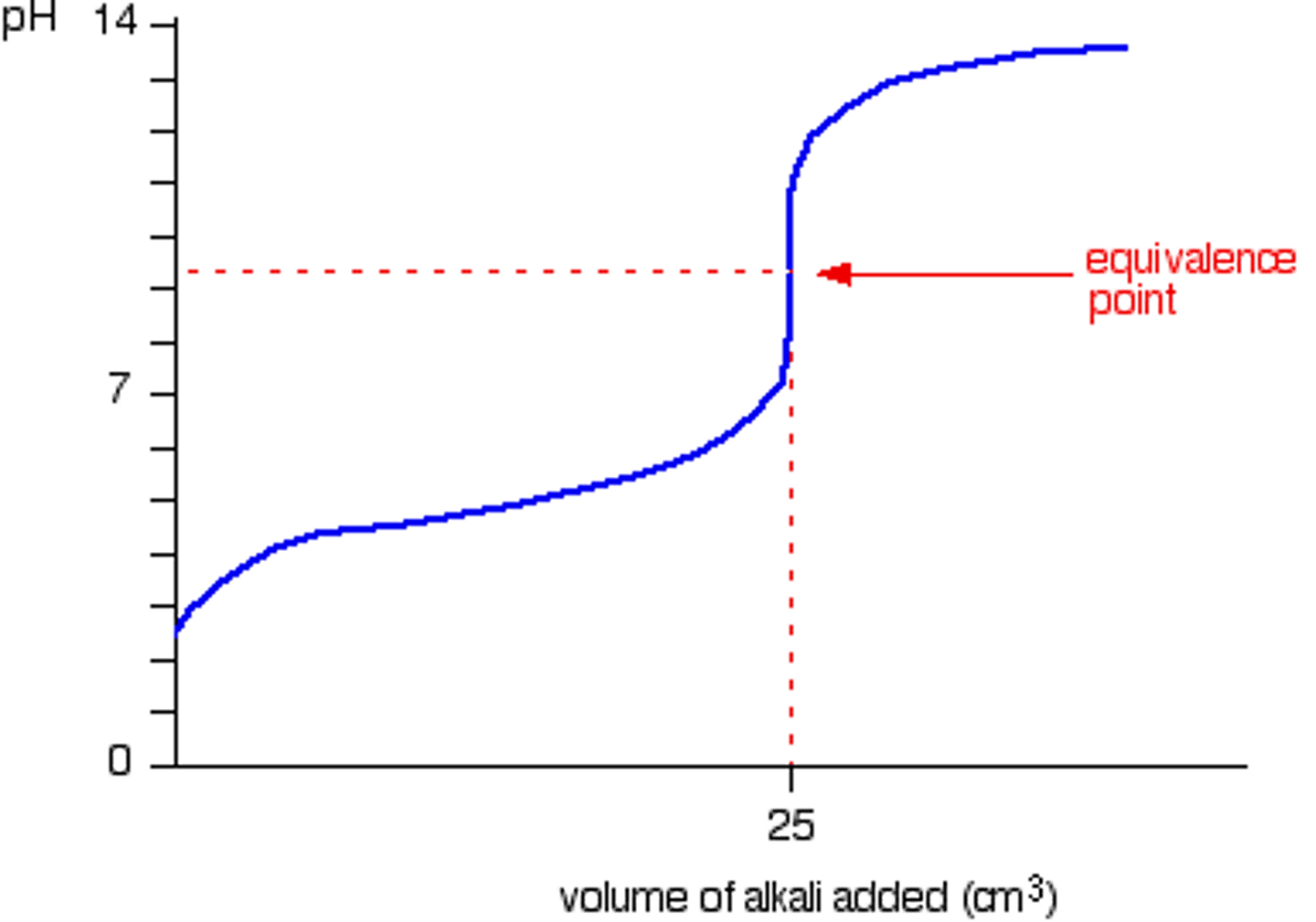
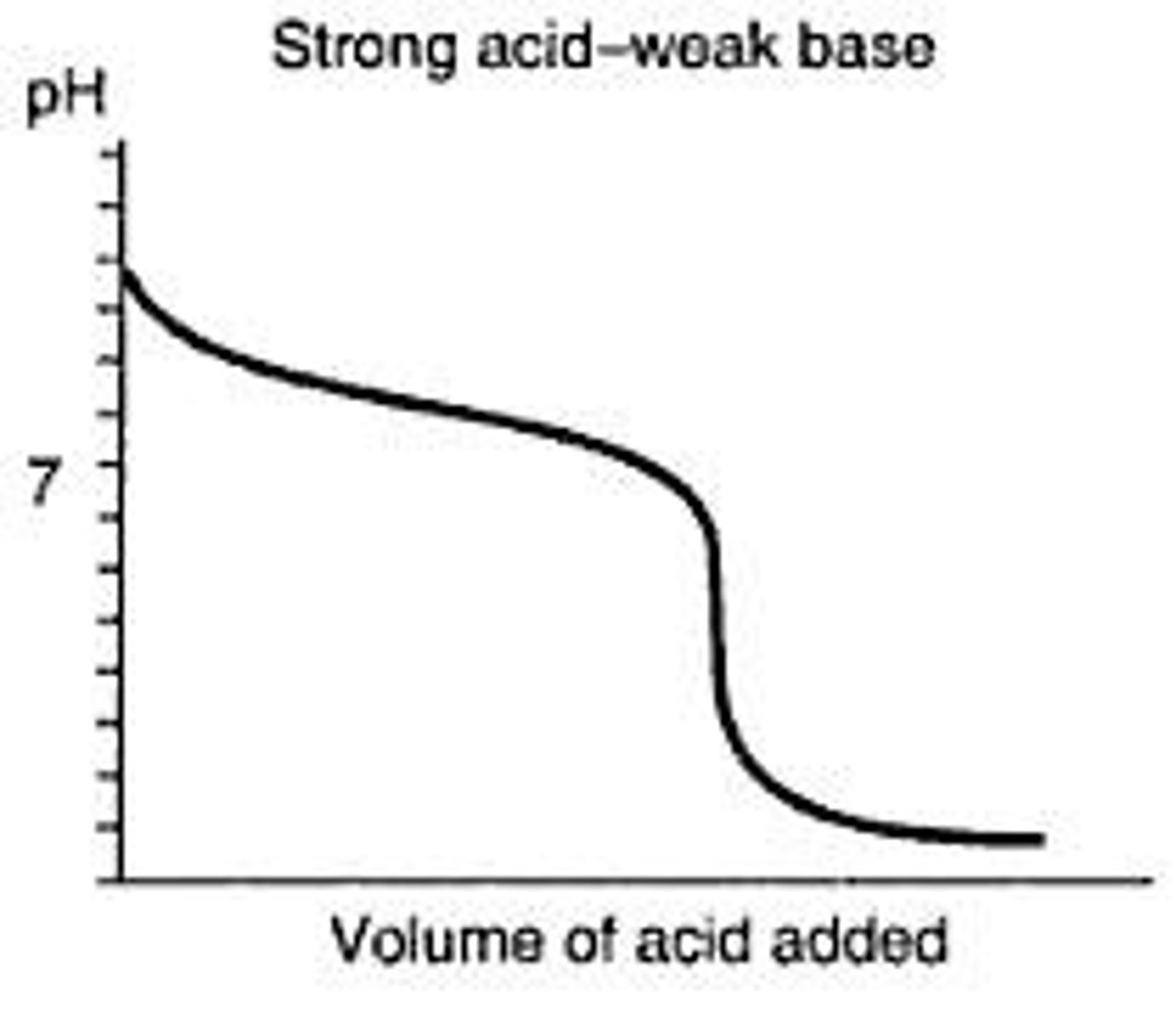
CRB Fill in the blank: For a weak base titrated with a strong acid, the equivalence point will occur at ________.
(A) pH > 7
(B) pH = 7
(C) pH < 7
(D) Not enough information to determine
(C) pH < 7
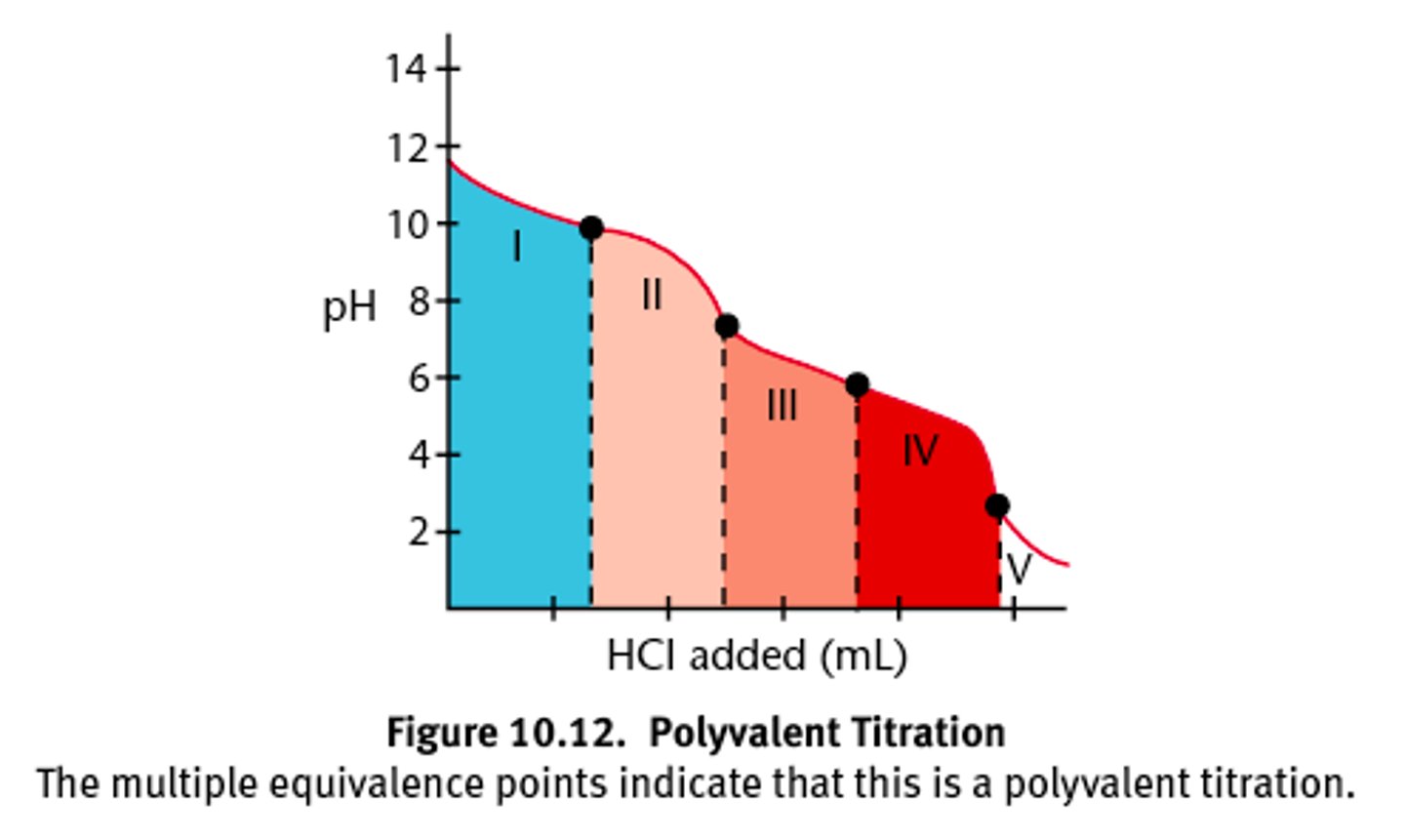
CRB True or false? For a polyvalent acid or base, there will be one elongated titration curve and a single endpoint.
False. For a polyvalent acid or base, there will be an endpoint for each of the acid or base equivalents, and typically some separation between one endpoint and the next titration.
What is the purpose of a redox titration vs. an acid/base titration?
THEY HAVE THE SAME PURPOSE! ;) The purpose is to find the concentration of a solution. The difference between these methods is not their purpose but their methodology. One uses the equivalence point between an acid and its base while the other uses the equivalence point between an oxidized species and its reduced species.
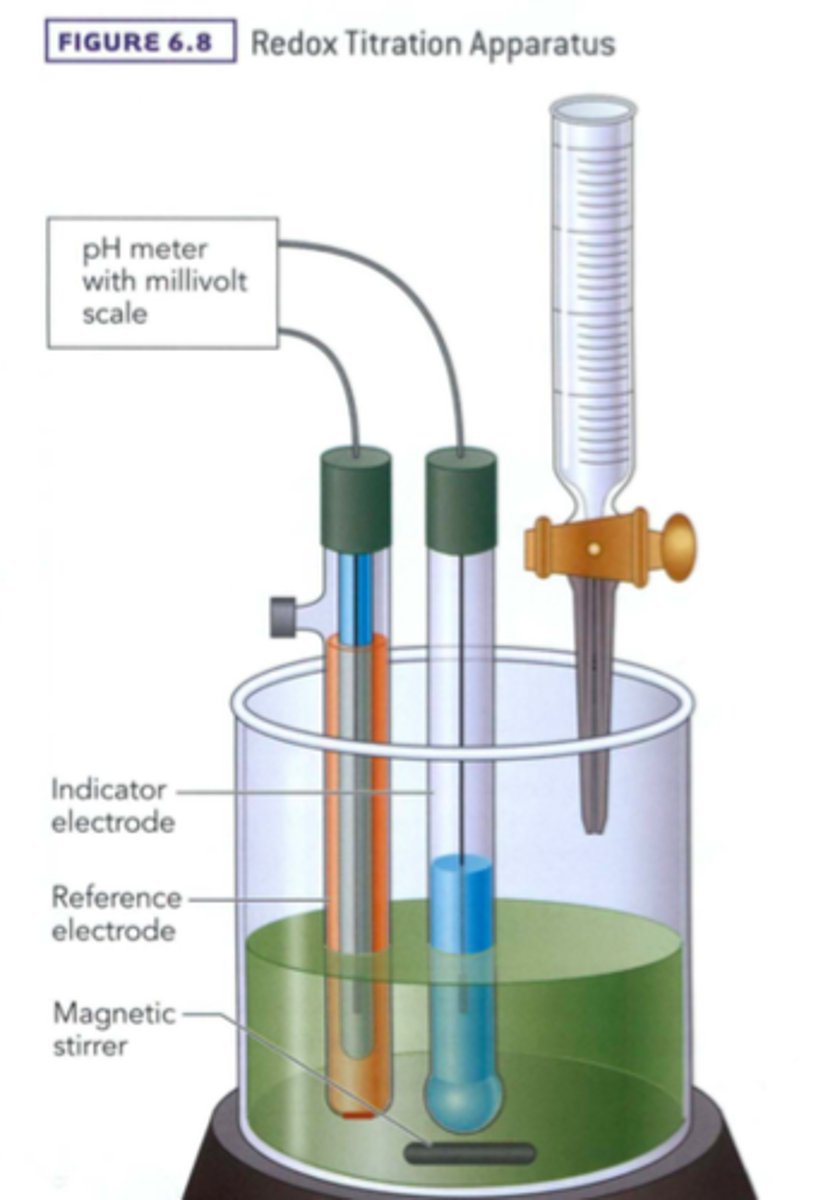
At the equivalence point, what is true concerning the number of moles added of the reducing vs. oxidizing species?
The number of moles added are such that no reactant remains when the reaction proceeds fully. This is always true about equivalence points.
Consider the following unbalanced reaction that results from titrating a 24.3 mL solution of Fe2+ with 67.9 mL of a 5.63 ⋅ 10^-3 M solution of KMnO4: MnO4- + Fe2+ -> Mn2+ + Fe3+ What is the concentration of the original Fe2+ solution?
(A) .0786 M
(B) .0324 M
(C) .9425 M
(D) .3652 M
(A) .0786 M
First, balance the reaction as follows: MnO4- + 5Fe2+ + 8H+ -> Mn2+ + 5Fe3+ + 4H2O
67.9 mL MnO4- ⋅ 1 L / 1000 mL ⋅ 5.63 ⋅ 10^-3 moles MnO4- / 1 L MnO4- = approx. 385 ⋅ 10^-6 moles MnO4-
(actual: 382.277 ⋅ 10^-6)
385 ⋅ 10^-6 moles MnO4- ⋅ 5 moles Fe2+ / 1 mole MnO4- = approx. 2000 ⋅ 10^-6 moles Fe2+ (actual: 1911 ⋅ 10^-6)
2 ⋅ 10^-3 moles Fe2+ / 2.43 ⋅ 10^-2 L Fe2+ = approx. 1 ⋅ 10^-1 M (actual: .786 ⋅ 10^-1)
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
What is the chemical structure of the following cation: ammonium?
NH4+
What is the chemical structure of the following anion: acetate?
CH3COO-
What is the chemical structure of the following anion: cyanide?
CN-
What is the chemical structure of the following anion: hydroxide?
OH-
What is the chemical structure of the following anion: permanganate?
MnO4-
What is the chemical structure of the following anion: nitrate?
NO3-
What is the chemical structure of the following anion: nitrite?
NO2- (it is more "lite" than NO3-)
What is the chemical structure of the following anion: perchlorate?
ClO4-
What is the chemical structure of the following anion: chlorate?
ClO3-
What is the chemical structure of the following anion: chlorite?
ClO2-
What is the chemical structure of the following anion: hypochlorite?
ClO-
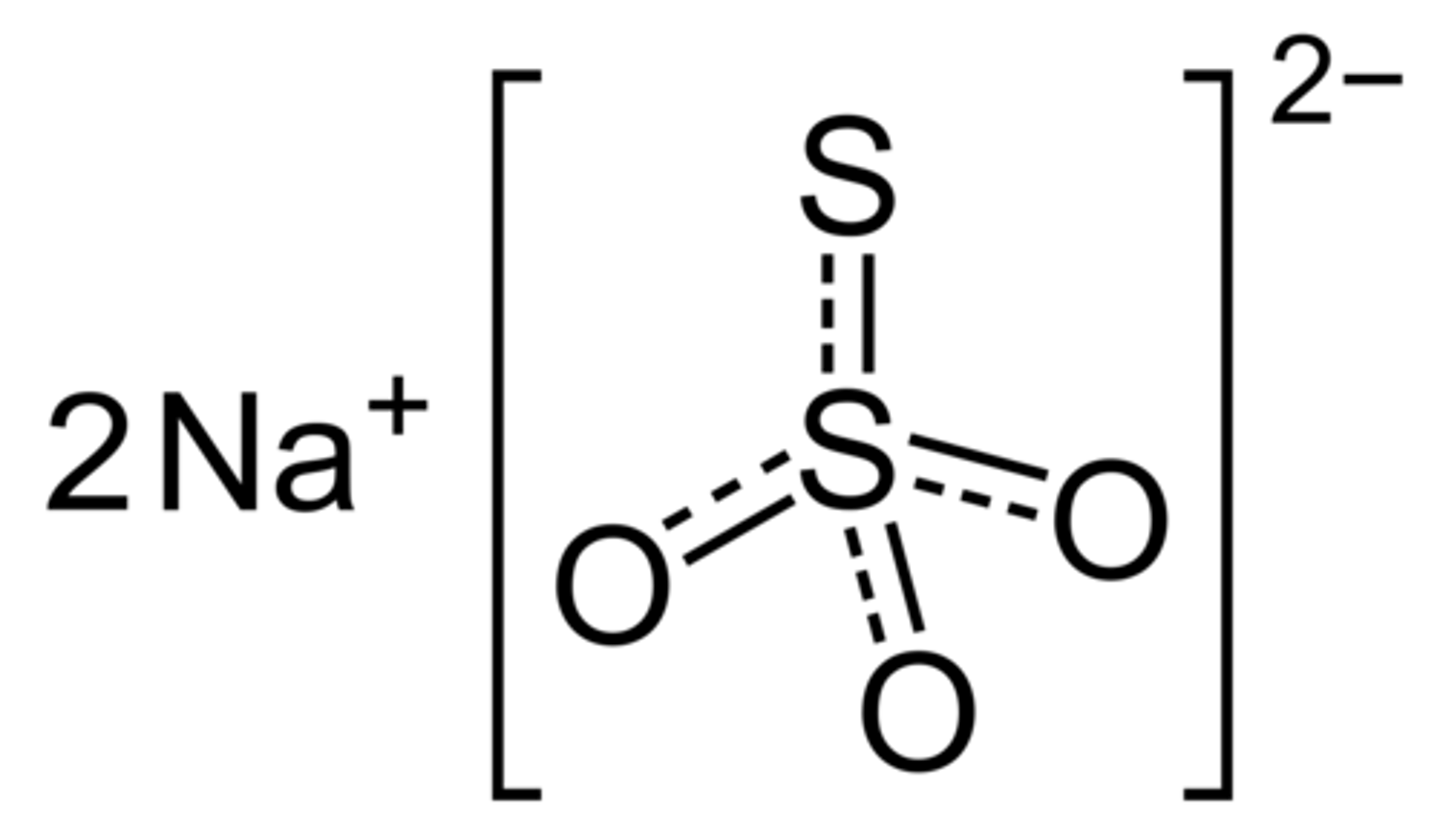
What is the chemical structure of the following anion: sulfate?
SO4(2-)
What is the chemical structure of the following anion: sulfite?
SO3(2-)
What is the chemical structure of the following anion: Hydrogen sulfate (bisulfate)?
HSO4-
What is the chemical structure of the following anion: carbonate?
CO3(2-)
What is the chemical structure of the following anion: Hydrogen carbonate (bicarbonate)?
HCO3-
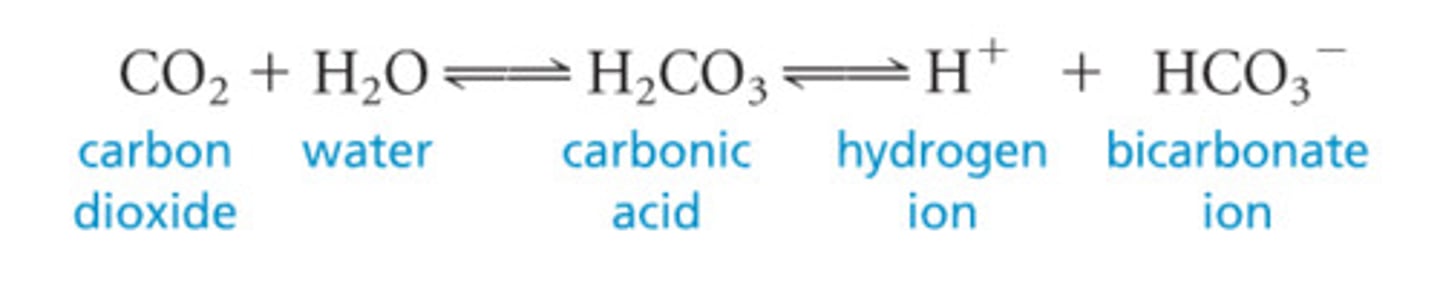
CRB Which of the following is not a key component of the bicarbonate buffer system, essential for controlling the pH in the blood?
(A) Carbonic acid
(B) H+
(C) Oxygen
(D) Water
(C) Oxygen
The bicarbonate buffer system relates carbon dioxide, water, carbonic acid, H+ and bicarbonate.
What is the chemical structure of the following anion: phosphate?
PO4(3-)
What is the chemical structure of the following anion: Hydrogen phosphate?
HPO4(2-)
What is the chemical structure of the following anion: dihydrogen phosphate?
H2PO4-
What is the chemical structure of the following anion: chromate?
CrO4(2-)
What is the chemical structure of the following anion: dichromate?
Cr2O7(2-)
What is the chemical structure of the following anion: oxalate?
C2O4(2-)
What is the chemical structure of the following anion: peroxide?
O2(2-)
What is the chemical structure of the following anion: thiocyanate?
SCN-
What is the chemical structure of the following anion: thiosulfate?
S2O3(2-)
When salt is dissolved in water, the Cl- anions become surrounded by water's Hydrogens or Oxygens? What about the Na+ cations?
Cl- anions are surrounded by the Hydrogens and Na+ cations are surrounded by the Oxygens.
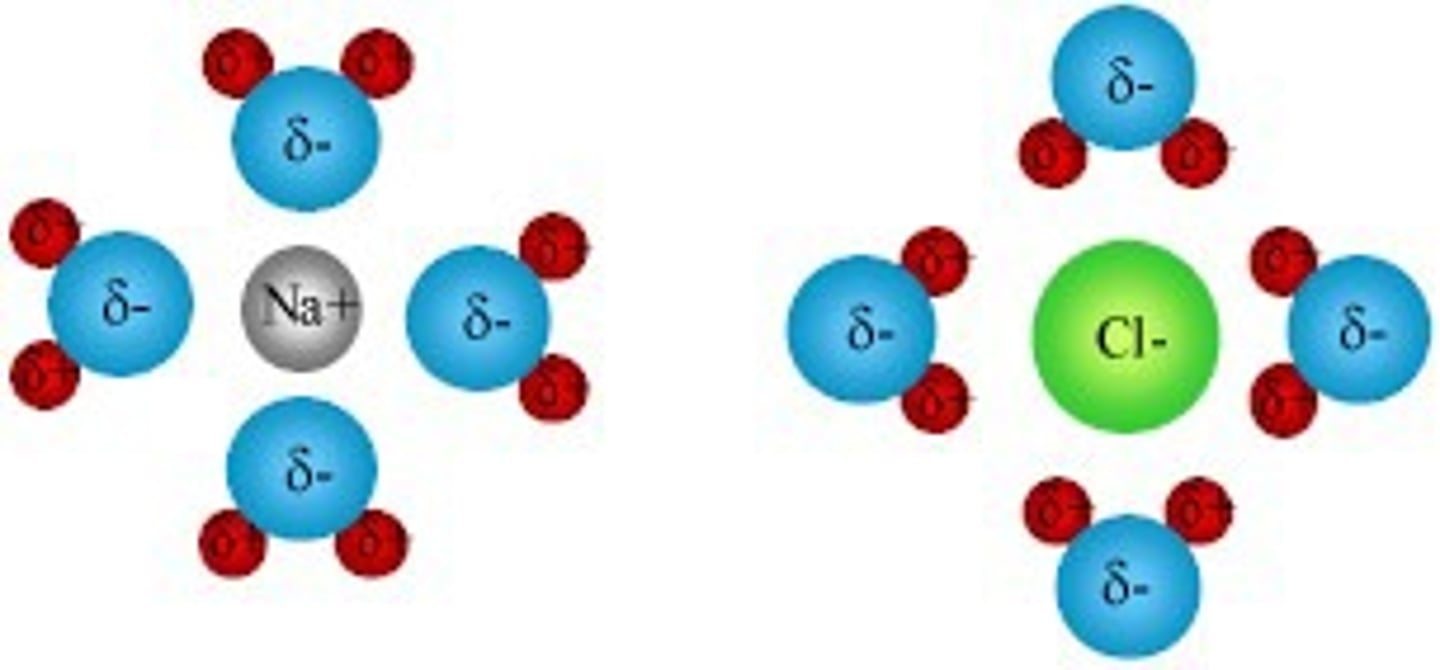
What process is the opposite of dissolution?
Precipitation is the opposite of dissolution.
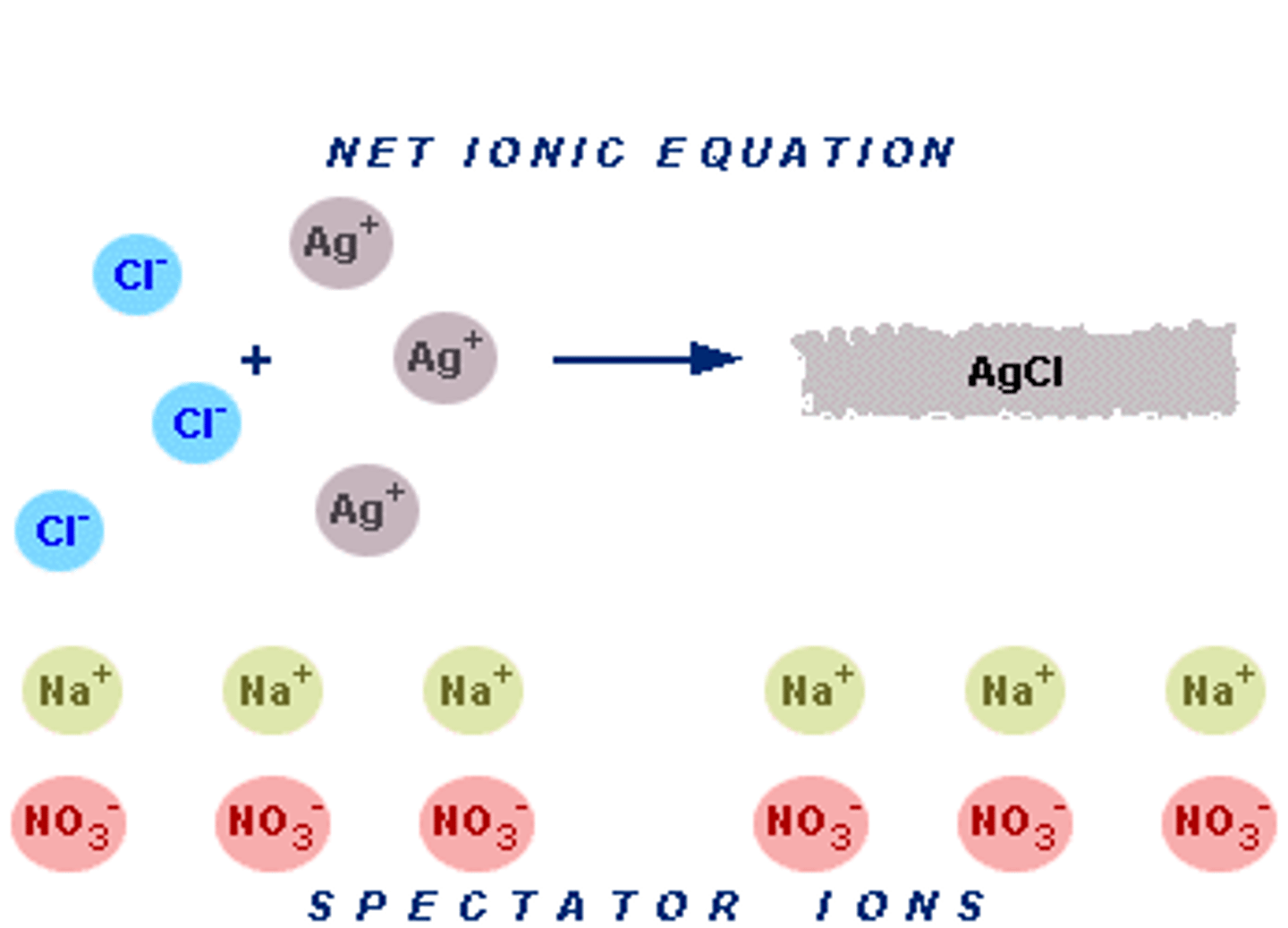
Write the net ionic equation for adding a solution of NaCl to a solution of AgNO3 to form the precipitate AgCl.
Ag+(aq) + Cl-(aq) -> AgCl(s)
Why are Na+(aq) and NO3-(aq) termed "spectator ions" in this case?
Because they do not participate in the reaction. They are merely watching ("spectating").
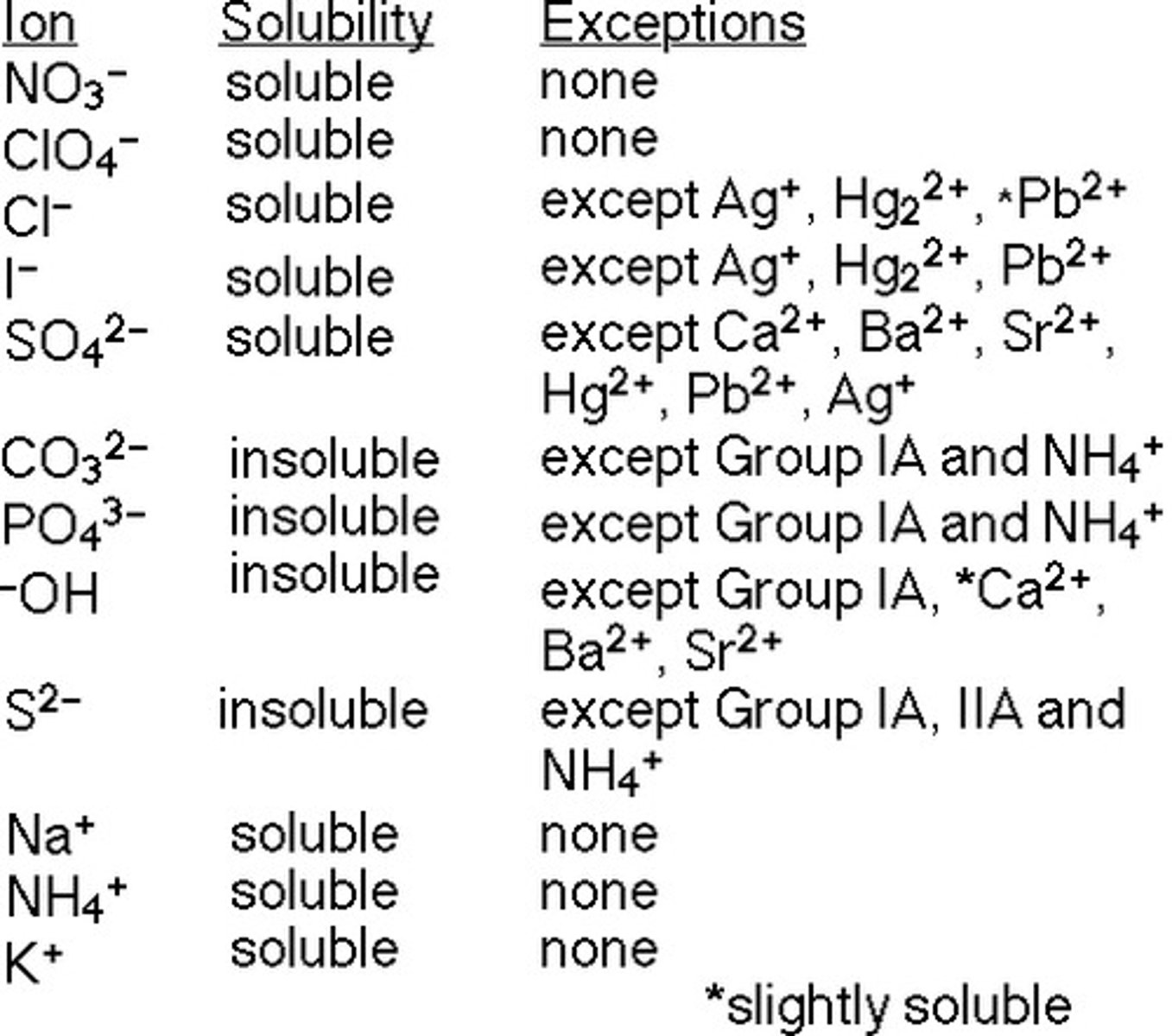
CRB Which of the following is NOT a salt solubility rule in water?
(A) All group 1 and ammonium salts are soluble
(B) All nitrate, perchlorate and acetate salts are soluble
(C) All carbonate and phosphate salts are soluble
(D) All silver, lead and mercury salts are insoluble, except for their nitrates, perchlorates and acetates
(C) All carbonate and phosphate salts are soluble
Carbonate and phosphate salts are typically INSOLUBLE, unless they are bound to group 1 or ammonium salts.
When you add a solution of NaCl to a solution of AgNO3, why is it that the precipitate is AgCl but not NaNO3 in terms of Ksp?
Because AgCl has a lower Ksp value, meaning it is more likely to form a precipitate at a lower concentration.