Midterm 2: Lipolysis, B-oxidation, and Lipogenesis & Ultraprocessed Food and the Brain
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
58 Terms
Overview of Lipid Catabolism
● When does this catabolism happen?
○ Interprandial State and/or Exercise
● The body adapts to low glucose by using stored fat for energy through lipolysis, β-oxidation, and ketone metabolism.
Lipid Catabolism Types
1) Lipolysis: Triglycerides → 3 free fatty acids + 1 glycerol
○ Primarily in adipose tissue
2) β-Oxidation: Fatty acids → acetyl-CoA molecules
○ Long chain fatty acids (LCFA, 13-21 C) oxidation: mitochondrial matrix
○ Very long chain fatty acids (VLCFA, 22 C): peroxisomes
○ The heart relies primarily on β-oxidation for energy, even at rest
3) Ketogenesis: During prolonged fasting, the liver converts excess acetyl-CoA into ketone bodies.
4) Ketolysis: The brain and skeletal muscle use ketones as an alternative fuel when glucose is limited.
Lipolysis Definition
● Definition:
Breakdown of triglycerides into 3 FFAs and 1 glycerol.
○ FFAs undergo β-oxidation in mitochondria
○ Glycerol is sent to the liver for TG synthesis or gluconeogenesis
● Location:
Cytosolic lipid droplets of adipose tissue
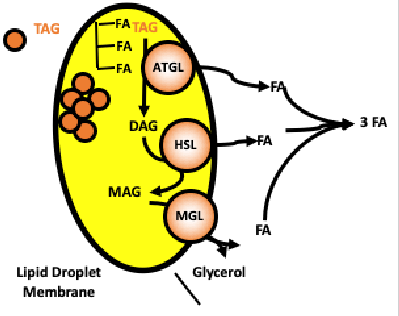
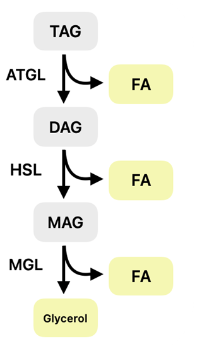
Lipolysis Enzymes
● Adipose Triglyceride Lipase (ATGL)
○ Initiates breakdown of triacylglycerol (TGs) to diacylglycerol (DAG) + FFA
● Hormone-Sensitive Lipase (HSL)
○ Hydrolyzes DAG to monoacylglycerol (MAG) + FFA
● Monoglyceride Lipase (MGL)
○ Converts MAG to glycerol + FFA
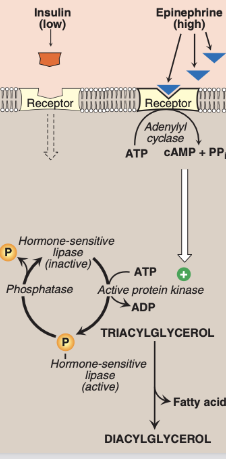
Regulation of Lipolysis through hormones
● Adrenalin activates lipolysis
○ Via activating Beta Adrenergic Receptors
○ Pathway: GPCR/AC/cAMP/PKA
■ Phosphorylates HSL, which increases lipolysis
● Insulin inhibits lipolysis
○ Via activating phosphodiesterase (PDE)
○ PDE degrades cAMP
○ Lowered cAMP disrupts the cascade
*HSL can convert TAG to DAG, but primarily converts DAG to MAG. HSL is considered more tightly regulated than ATGL due to extensive controls, including phosphorylation by PKA
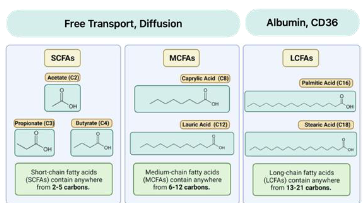
Fatty Acid Transport
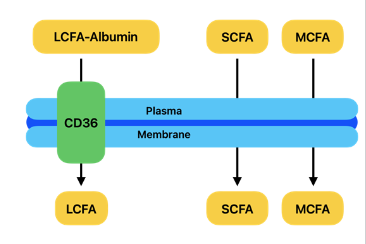
● FFA (LCFA: large chain fatty acids) transport via albumin
○ After leaving adipocytes, hydrophobic FFAs bind to albumin to travel in the blood
○ Albumin ensures FFAs reach tissues for energy production
● SCFAs and MCFAs(small and medium chain fatty acids) are smaller, and can travel freely through blood without the need for albumin
○ Also cross plasma membranes and mitochondrial membranes freely
Fate of Glycerol
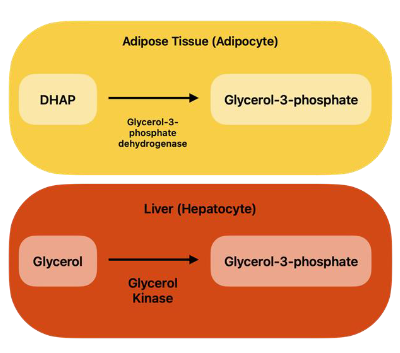
● Adipocytes lack glycerol kinase, so glycerol is sent to the liver.
● Liver: Glycerol kinase converts glycerol to glycerol-3-phosphate (G3P) for TG synthesis, GNG.
● Adipocytes: Glycerol-3-phosphate dehydrogenase converts DHAP to glycerol-3-phosphate for TG synthesis
Fatty Acid Uptake
• CD36 mediates FA entry into the cell
○ Stimulated by insulin and exercise (in different conditions)
• Increased CD36 translocation enhances the cellular uptake of fatty acids along concentration gradient.
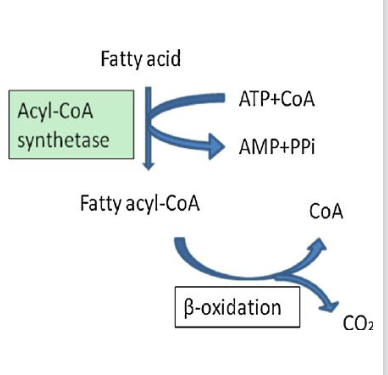
Fatty Acid Activation to synthesize acyl CoA
Acyl-CoA Synthetase (ACS) synthesizes fatty acyl CoA
o Addition of CoA required for FA metabolism since CPT1 transports FA-CoA, not FA
o ACS is present in three locations within cell
■ Plasma membrane
■ Outer mitochondrial membrane
■ Endoplasmic reticulum membrane
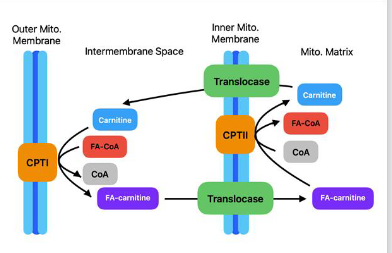
Carnitine Shuttles for FA (LCFA) Uptake
● CPT-I (Carnitine palmitoyltransferase I)
○ Location: present on outer mitochondrial membrane
○ Function: Converts FA-CoA to FA-Carnitine
○ RLS: CPT-I is the rate-limiting step of β-oxidation
○ Regulation: Inhibited by malonyl-CoA.
● CPT-II (Carnitine palmitoyltransferase II)
○ Location: Present on outer mitochondrial membrane
○ Function: Converts FA-carnitine to FA-CoA(Opposite of CPT-1)
Importance of Carnitine in FA (LCFA) Transport
● Carnitine Shuttle: Transfers LCFA-CoA into the mitochondria.
○ Carnitine deficiencies impair LCFA metabolism (LCFAs cannot enter mitochondria)
● Carnitine can be synthesized from amino acids in liver and kidney
● However, other tissues (heart, skeletal muscle, etc.) cannot
○ Depend on diet for carnitine
○ Sources: meat, milk, avocado, other foods
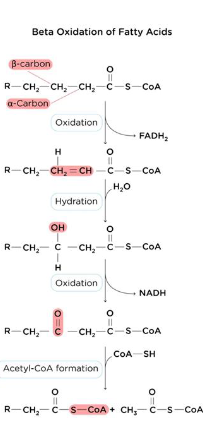
β-Oxidation Overview(and total round equation for even # carbon fatty acids)
• Mitochondrial process that removes 2-carbon fragments from FA-CoA per cycle
• Repeats until FA fully converted to acetyl CoA molecules, NADH, and FADH2
• Total rounds of β-Oxidation
○ (N/2) - 1 rule (for FA with even number of carbons)
○ Example: (16/2) - 1 = 7 rounds
β-Oxidation Key Enzymes
1) Acyl CoA dehydrogenase: Oxidation
○ Forms trans double bond between alpha and beta carbons
○ Produces FADH2
○ Family enzymes specific to chain length (LCAD, MCAD, SCAD)
2) Enoyl-CoA Hydratase: Hydration
○ Adds a hydroxyl group to the beta carbon
○ Adds proton to alpha carbon
○ No energy direct production
3) 3-hydroxyacyl CoA Dehydrogenase: Oxidation
○ Oxidizes beta carbon
○ Produces NADH
4) β-Ketoacyl-CoA Thiolase: thiolytic cleavage
○ Cleaves bond between alpha and beta carbons
○ Produces fatty acyl CoA (n-2)
Regulation of β-Oxidation
● Product Inhibition
○ Each enzyme in β-oxidation is inhibited by the specific acyl-CoA intermediate it produces.
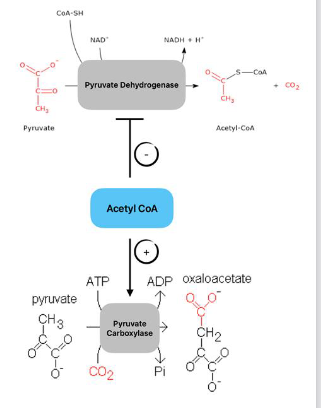
● Acetyl-CoA
○ Stimulates pyruvate carboxylase (promotes gluconeogenesis).
○ Inhibits pyruvate dehydrogenase (limits excess acetyl-CoA production).
● High NADH/NAD⁺ ratios
○ A signal of high energy
○ Inhibits β-oxidation
● Malonyl-CoA
○ Strongly inhibits CPTI, the RLS of β-oxidation
○ Prevents simultan
Energy Yield from β-Oxidation
● Complete oxidation of palmitate (16C) to CO2 and H2O produces:
○ 8 acetyl CoA, 7 NADH, 7 FADH2 = 129 ATP (net)
■ FADH2 corresponds to 1.5 ATP in ETC
■ NADH corresponds to 2.5 ATP in ETC
○ But 131 ATP total (2 ATP required by ACS for activation of FA)
Odd-numbered FA & Unsaturated FA
● Unsaturated FA(double bond FA)
○ Produce less energy than equivalent length saturated FA(single bonds FA)
○ Monounsaturated Fatty Acids (e.g., Oleic acid 18:1):
■ Needs 3,2-enoyl CoA isomerase and enoyl CoA hydratase for conversion
○ Polyunsaturated Fatty Acids (e.g., Linoleic acid 18:2):
■ Requires 2,4-dienoyl CoA reductase + isomerase
● VLCFA
○ Initially processed via β-Oxidation in peroxisomes
● Branched chain FA
○ α-Oxidation
● Odd-numbered saturated FA
○ Regular β-oxidation continues until a 3-carbon molecule, propionyl CoA, remains
○ Propionyl CoA is converted to succinyl CoA by propionyl CoA carboxylase and other enzymes, allowing it to enter the TCA cycle
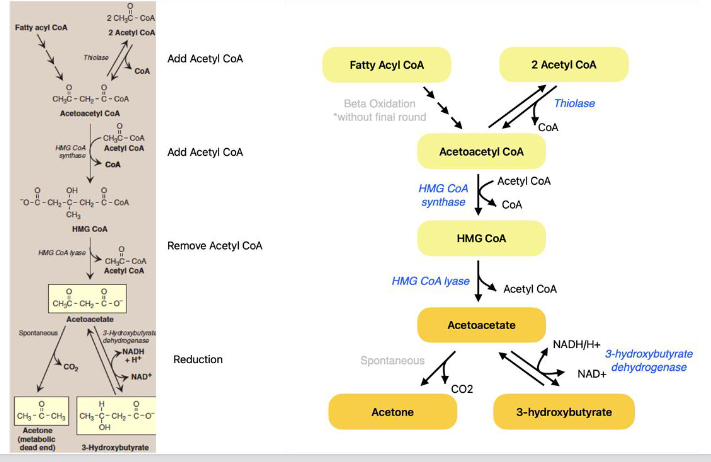
Ketogenesis
● The liver produces three ketone bodies: acetoacetate, β-hydroxybutyrate, and acetone
○ Acetone escapes via breath
● When: Fasting, prolonged exercise, or low-carb diets
● Steps:
○ Thiolase: produces acetoacetyl-CoA
○ HMG-CoA synthase: produces HMG-CoA
■ rate-limiting enzyme
○ HMG-CoA lyase: produces acetoacetate + acetyl-CoA
○ 3-hydroxybutyrate dehydrogenase
○ Acetoacetate can be converted to 3-
hydroxybutyrate or spontaneously to acetone
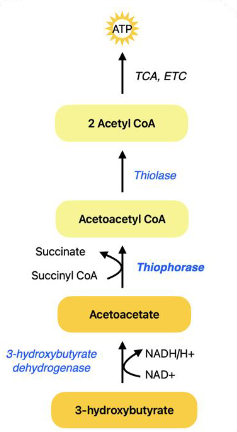
Ketolysis
● Ketone bodies (acetoacetate, 3-hydroxybutyrate) converted back into acetyl-CoA in muscles and brain
● Key Enzymes
○ 3-hydroxybutyrate dehydrogenase: converts 3-hydroxybutyrate
to acetoacetate
○ Thiophorase: converts acetoacetate to acetoacetyl-CoA
■ Also called: succinyl CoA:acetoacetate CoA transferase
■ Liver lacks thiophorase, which ensures ketones are only utilized by extrahepatic tissues
○ Acetoacetyl-CoA converted to 2 acetyl-CoA molecules for ATP production
Diabetic Ketoacidosis (DKA)
● Occurs in Type 1 Diabetes due to lack of insulin
○ Uncontrolled lipolysis, β-oxidation, and ketogenesis
○ Insulin inhibits lipolysis by reducing HSL activity
● Results in high ketone levels, leading to acidic blood (pKa of ketones ≈ 4)
● Many negative physiological consequences (enzyme impairment, CNS issues, etc.)
Pros and Cons of Lipid Metabolism
● Pros
○ Highest energy value per gram (9 kcal/g)
○ Can be stored in many sites
○ Stable and compact (not stored with water)
● Cons
○ Depends on oxygen (must be metabolized via oxidative metabolism)
○ Yield less energy / O2 used (kcal per L O2)
○ Slow - require mobilization and transport from AT into the mitochondria of other tissues
Conclusion of Lipid Catabolism(lipolysis)
● This catabolism happens under fasting, prolonged exercise, or carbohydrate scarcity
○ Lipolysis, β-oxidation, and ketogenesis allow the body to utilize stored fats
● Key Takeaways
○ 1. Lipolysis releases free fatty acids for energy
○ 2. β-Oxidation produces acetyl-CoA for ATP or ketone production.
○ 3. Ketone bodies serve as an alternative energy source during glucose deprivation
Lipogenesis - General Concepts
• Lipogenesis or fatty acid de novo synthesis is the process of synthetizing fatty acids from acetyl-CoA
• In principle, it could take place in all cells
o Primarily occurs in the liver and lactating mammary glands
o To a lesser extend in the adipose tissue and muscles
• Takes place in the cytosol
• Incorporates carbons from the acetyl-CoA into the growing fatty acid chain
• Requires ATP and NADPH
• Anabolic process that occurs when the body has an excess of carbohydrates and energy, converting these nutrients into fatty acids and eventually triglycerides for storage
Lipogenesis – Fatty Acid Synthesis
Fatty acid synthesis
1) Glucose derived from dietary carbohydrates undergoes glycolysis and TCA cycle to produce citrate in the mitochondria.
2) Citrate is transported in the cytosol and converted into acetyl-CoA by ATP-citrate lyase (ACLY).
3) Acetyl-CoA is converted to malonyl-CoA by acetyl-CoA carboxylases 1 (ACC1).
4) Fatty acid synthase (FAS), condenses acetyl-CoA and malonyl-CoAs into palmitate, which is the first fatty acid product.
5) Palmitate undergoes the elongation and desaturation reactions to generate complex fatty acids
Lipogenesis – Fatty Acid Synthesis Step 1: Production of cytosolic acetyl-CoA
Step 1: Production of cytosolic acetyl-CoA
The first step is the transfer of acetate units from mitochondrial acetyl CoA to the cytosol.
The CoA portion of acetyl-CoA, however, cannot cross the inner mitochondrial membrane; only the acetyl portion enters the cytosol.
→ Acetyl-CoA combines with oxaloacetate to form citrate which is transported outside the mitochondrion and then reconverted by the ATP-citrate lyase into Acetyl-CoA and OAA.
This process only happens when citrate concentration is high as a consequence of isocitrate dehydrogenase (step 3 TCA) inhibition by the presence of large amounts of ATP.
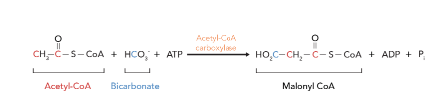
Lipogenesis – Fatty Acid Synthesis Step 2(formation of Malonyl-CoA)
Step 2: Formation of Malonyl-CoA Enzyme: acetyl-CoA carboxylase (ACC)
Acetyl-CoA is carboxylated to malonyl-CoA by AAC
This reaction requires ATP and biotin as a cofactor.
This is the rate-limiting step of lipogenesis as well as the regulated step of FA synthesis.
Malonyl-CoA serves as the two-carbon donor unit for the elongation of the fatty acid chain
Regulation of AAC in Fatty Acid Synthesis
1) Short-term regulation
• Direct allosteric regulation: Activation by citrate, which causes dimers to polymerize, and inactivation by LCFA-CoA (pathway’s end product) which causes its depolymerization
• Reversible phosphorylation: AMPK phosphorylates and inactivates ACC. AMPK itself is allosterically activated by AMP and covalently activated by phosphorylation via several kinases. Conversely protein phosphatase dephosphorylates and activates AAC.
→ This is the same mechanism that regulates glycogen synthase
2) Long-term regulation (more on this later)
Expression levels of AAC are affected by the diet
High Carbohydrate diets → increase in AAC levels
Low carbohydrate diets → decrease in AAC levels
Further Elongation & Chain Desaturation
Palmitate (16-carbon fully saturated LCFA) is the primary end product of FAS activity.
However, palmitate (Hexadecanoic; 16:0) can undergo a number of modifications, resulting in desaturation and/or elongation.
Further elongation
Elongation to stearate (Octadecanoic;18:0) mainly occurs in the SER.
Same steps as FAS, but the 4 successive steps of the elongation are
performed by individual enzymesSome tissues, like the brain have extra elongation capabilities and can
generate VLFA (i.e. 22+ C)
Desaturation:
Fatty Acyl-CoA desaturases, also present in the SER are responsible for
desaturating LCFA (i.e. adding cis double-bond)The desaturation reaction requires O2 and NADH
The first double-bond is generally inserted between carbon 9-10 by the Δ9- desaturase → Oleic acid (18:1 (9)) and small amounts of palmitoleic acid (16:1 (9)). Additional double bonds can be added by different desaturases.
Human have 9, 6, 5 and 4 desaturases → cannot introduce from double bonds form the
10 to the ω end of the chain
Triglyceride Synthesis and Storage(and G3P involvement)
The synthesized fatty acids are esterified with glycerol-3-phosphate to form triglycerides
The 3 FAs that form the TGs are usually not the same:
1) Saturated
2) Unsaturated
3) Either
Two pathways for Glycerol 3-phosphate synthesis(initial acceptor of FAs):
Produced from glucose (liver and adipose tissue)
Glycerol kinase convert free glycerol directly into Glycerol 3-phosphate (liver only)
Triglyceride Synthesis and Storage
Fatty acid activation
Acyl-CoA Synthetase (ACS) convert fatty acids into FA-CoA (active form)
Triglyceride synthesis - set of 4 reactions
1)Acyltransferase (x2): addition of 2 fatty acids to a molecule of glycerol phosphate
2) Removal of the phosphate group by phosphatase
3) Addition of the 3rd fatty acid
TG fate in liver and adipose tissue:
*In adipose tissue → TGs are stored as fat droplets in the cytosol (TGs in its core + phospholipid monolayer on its surface)
*In the liver → little storage → most is packaged into VLDL(Very low density lipoprotein) → secreted into the bloodstream
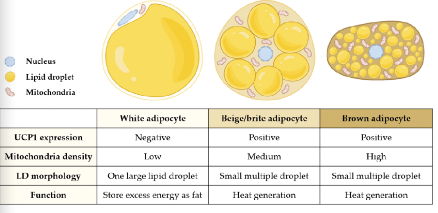
The Adipocyte(s)
WAT is divided in subcutaneous adipose tissue (sWAT) and visceral adipose tissue (sWAT)
Beige adipocytes reside within WAT (mostly inguinal) and can express UCP-1 (following e.g cold exposure)
→ Flexible! Can acquire a thermogenic or storage phenotype, depending on environmental cues(Once an adipocyte is locked into a phenotype, can’t change back)
BAT is transcriptionally similar to muscle tissue and expresses high levels of UCP-1 → heat!
Increase in fat mass:
1) In the early stages of fat accumulation →Hypertrophy (increase in adipocyte size)
2) When max capacity is reached → Hyperplasia (increase in adipocyte number) via differentiation of pre-adipocytes*stem cell-like precursors) into mature adipocytes.
WAT is not that metabolically active (5-10% of resting EE, same as the kidneys, which weigh ~80-220g each)
Lipogenesis - General Regulation(transcription Regulation)
In addition to allosteric, covalent and product regulatory mechanisms...
Many of the enzymes involved in DNL are regulated also (primarily) at the transcriptional level in a coordinated manner.
Transcriptional activation of these lipogenic genes after a carbohydrate-rich meal can be achieved a set of three transcription factors:
• Sterol regulatory element-binding proteins (SREBPs)
• Carbohydrate-responsive element-binding proteins
(ChREBPs)
• Liver X receptors (LXRs)
Pharmacological inhibition of Lipogenesis and diseases associated with Lipogenesis
Although DNL is vital to maintain whole-body and cellular homeostasis, chronic elevations are associated with the development of a broad spectrum of diseases and disorders
o Cardiovascular disease (CVD)
o Nonalcoholic fatty liver disease (NAFLD)
o Type 2 diabetes
o Obesity
o Numerous cancers
Several natural (e.g. cucurbitacin B, abundant in cucumber) and synthetic DNL antagonists are currently being tested
Still many important questions that remain to be answered!
- Unclear whether systemic or organ-specific inhibition of DNL
should be targeted
- What degree of inhibition is necessary to avoid potential side
effects such as defects in fetal development, muscle disfunction, etc
Energy Requirement
ACC requires 1 ATP per malonyl-CoA formed
To synthesize palmitate, 8 acetyl-CoA molecules are used, but 7 of them must be converted to malonyl-CoA.
→ 7 ATP are required
If one starts from citrate, one must add another 8 ATPs
Additionally, it requires the conversion of 14 high-energy NADPH molecules to NADP+. NADPH production via the PPP is cost neutral but if NADPH is produced via the malic enzyme (malate → pyruvate)
Energy was invested in the form of 1 NADH to generate malate from oxaloacetate in the cytosol
Energy required for TG synthesis:
Activation of each FA: 2 ATP per FA → 6 per TG.
Formation of glycerol-3-phosphate: 1 ATP (via glycerol kinase) or NADH (via glycerol-3-phosphate dehydrogenase, if using DHAP).
→ Significantly more energetically demanding than just store dietary fat
Summary of Fat
The main fuel stored in the bodies of animals is fat. A young adult human's fat stores average between ~15–20 kg (~20% of BW), but varies greatly depending on age, sex, and individual disposition
In contrast, the human body stores only ~400 g of glycogen of which ~300 g is locked inside the skeletal muscles and is unavailable to the body as a whole.
Fatty acids are broken down to acetyl-CoA via beta-oxidation inside the mitochondria, whereas fatty acids are synthesized from acetyl-CoA outside the mitochondrion, (i.e. in the cytosol).
The two pathways are distinct, not only in where they occur, but also in the reactions that occur, and the substrates that are used.
The two pathways are mutually inhibitory, preventing the acetyl- CoA produced by beta-oxidation from entering the synthetic pathway via the acetyl-CoA carboxylase (Step 2).
What is Processed Foods
▪ “Processed food“: any food other than a raw agricultural commodity; includes any raw agricultural commodity that has been subject to processing, such as canning, cooking, freezing, dehydration, or milling
Essentially, food processing is defined as any procedure that alters food from its natural state
* "raw agricultural commodity" means any food in its raw or natural state, including all fruits that are washed, colored, or otherwise treated in their unpeeled natural form prior to marketing.
Levels of Processed Foods
Minimally processed foods: fruits and vegetables
Processed foods to help preserve and enhance nutrients and freshness of food at their peak(butter and dried fruits)
Ready to eat foods(ice cream)
Food packaged to stay fresh and save time(digiorno pizza)
Foods that combine ingredients such as sweeteners, spices, oils, flavors, color, preservatives to improve safety/taste(cake)

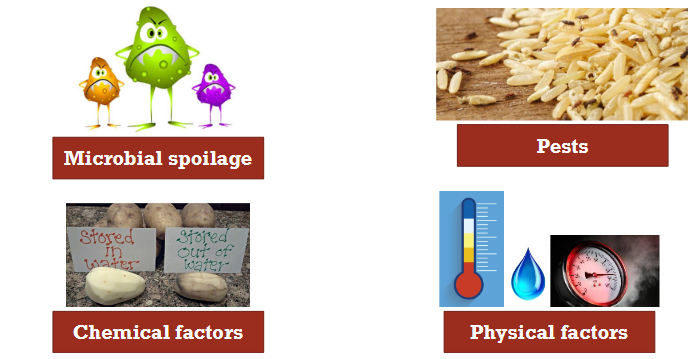
Why do we need to process food
To stop:
Microbial spoilage
chemical factors
pests
physical factors
Basic Types of Procedures for Food Preservation
1. Add heat
2. Remove heat
3. Preservatives and/or additives
4. Remove available water
5. Lower pH
6. Add barriers (packaging)
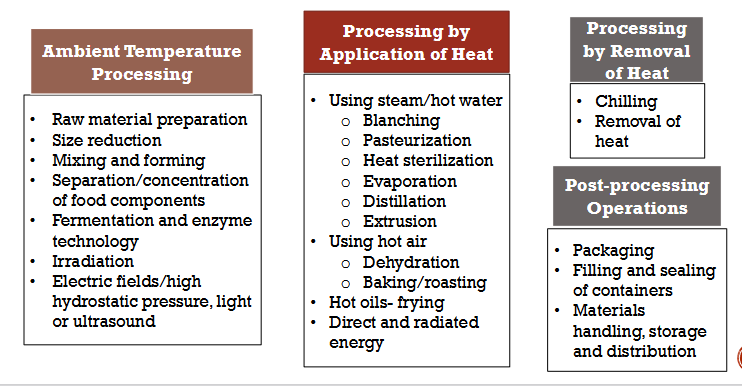
Food Processsing methods: Overview
Ambient Temperature Processing
Processing by Application of Heat
Processing by Removal of Heat
Post-processing Operations
Effects of Processing(advantage and disadvantage)
Addition of Heat
Advantage: Palatability, Microorganisms (reduction/kill)
Disadvantages: Nutritional properties, Sensory properties
removal of heat
Advantage: Microbial and enzymatic activity reduced, Storage life
Disadvantage: Frozen food-crystal size/texture, Energy intensive
Water removal
Advantage: Slows/prevents microorganism growth, Reduced shipping cost
Disadvantages: Alter appearance/texture, Nutritional quality, Energy intensive
Preservatives/additives:
Advantage: Shelf life improved. Organoleptic properties improved
Disadvantages: Consumer perception, Health concerns
The Nova Classification System for Processed Foods(Groups)
Group 1: Unprocessed or Minimally Processed Foods(eggs, grains, nuts, etc.)
Group 2: Processed Culinary Ingredients(Plant oils, aniimal fats, maple syrup)
Group 3: Processed Foods(canned vegetbales, meat, fish, fruit
Group 4: Ultra-Processed Foods(Sugar sweetened beverages, frozen dishes, reconssituetd meat products)
“Ultra-processed” foods constitute ~65% of total energy intake in children ages 2-19 in the U.S.(Neri et al., Pediatric Obesity, 2019)
Pros and Cons of Nova Classification System
▪ Pros:
➢Relatively easy to grasp basics▪ Cons:
➢Categorization of different food processes is sometimes arbitrary(e.g., grinding in group 2 vs. fermentation in group 3)
➢Some better-for-you foods can still be in group 4 (ultra-processed group), e.g., yogurt
➢Categorization of different foods into the groups can be subjective
➢Does not allow for mechanistic insights to be gained about the effects food processing
➢Is NOVA really just a different way to classify diet quality? (Western diet, palatability, energy density...)
The “processed” food dilemma
• Food processing / processed foods lack clear and robust definitions and classification, making it challenging to study the physiological and behavioral impacts of consuming “processed” foods
What we do know:
• Heat treatment is a ubiquitous food processing operation, promoting the Maillard reaction and ensuing formation of advanced glycation end products (AGEs)
➢ Major role in flavor• Evidence suggests AGEs are implicated in nephropathy, obesity, retinopathy,
neurodegeneration, and inflammation (Chaudhuri et al., Cell Metabolism, 2018; Snelson et al., Science Advances, 2021
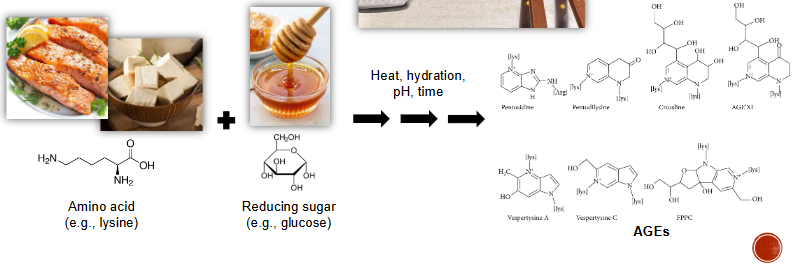
Mallard Reaction
Main heat reaction to create AGE
Amino Acid + reducing Sugar >(heat, hydration, pH, time)> AGEs
Quantification of AGEs in baked vs. unbaked diets by LC-MS
More MG-H1, CML, and CEL AGEs in baked food compared to unbaked food
This reaction is used to bake toast
Mallard Reaction and resulting high level of AGes effect on neurocognition study design and timeline
• Male Sprague Dawley rats with early life diet exposure:
o Control group receiving AIN-93G unbaked diet (UNBAKED, n=24)
o Experimental group receiving AIN-93G baked diet (BAKED, n=12)
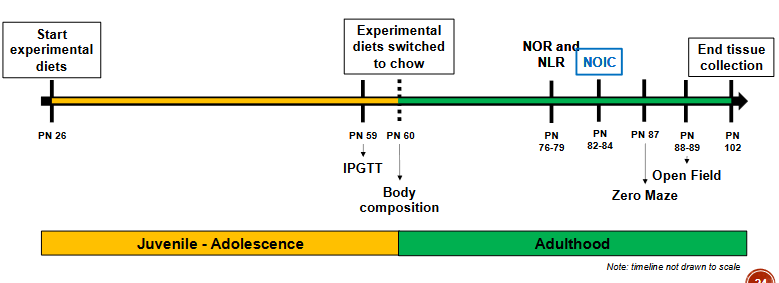
Hippocampal-dependent memory (NOIC: novel object in context) – contextual episodic memory in the experiment
3 Novel contexts, 1 and 2 are different context boxes with 1 having circle and square and 2 having two squares in box
Context: context 2 has one square replaced with circle, episodic memory would mean that the rat will notice the circle sooner.
Results demonstrated that baked BAKED group showed deficits in contextual episodic memory compared to CTL
Evaluating AGE-Specific Effects through the usage of drug that breaks down AGEs
• Male Sprague Dawley rats with early life diet exposure:
o Unbaked (UNBAKED, n=12)
o Unbaked plus 1mg/kg/day alagebrium in drinking water (UNBAKED+ALA, n=12)
o Baked (BAKED, n=12)
o Baked plus 1mg/kg/day alagebrium in drinking water (BAKED+ALA, n=12)
o A drug that breaks down AGEs prevents the memory impairments otherwise observed for a heat-treated diet
Mechanism of AGEs effect on brain(microbiome)
• Previous research has identified connections between early life diet consumption, neurocognition/memory, and the gut microbiome (Kendig et al., Appetite, 2022; Tsan et al., Nutr Neurosci, 2021; Noble et al., J Nutr, 2017; Noble et al., Trans Psych, 2021)
Microbiome Analysis
Highly significant differences in the abundance of Lactococcus that strongly correlated with NOIC performance
more lactococcus in unbaked food population vs baked food population
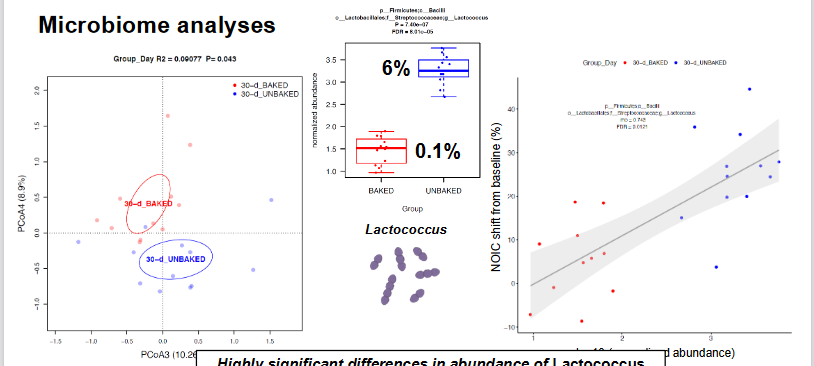
Looking into Lactococcus Procedure
Male Sprague Dawley rats with early life diet exposure:
o All rats received AIN-93G baked diet
➢ Rats were weaned onto baked chow at PN 21 (to reduce the likelihood that they would have Lactococcus from any other diet)
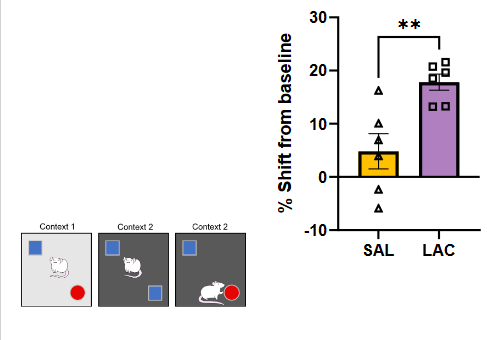
o Probiotic supplementation from PN 26-40:
➢ Gavages of saline given 3 days/week (SAL)
➢ Gavages of Lactococcus (200 μL of 109 CFU/mL per day) given 7 days/week (LAC)
o AGEs, memory, and Lactococcus
➢ Early life 2-week daily Lactococcus gavage (LAC) improved memory performance compared to 3 days/week saline gavage (SAL) among rats given early life diet high in AGEs.
Intermediate summary and conclusions: AGE-rich diet
• Consuming a heat-treated diet (Maillard reaction, AGE formation) during the juvenile and adolescent period resulted in impaired hippocampal-dependent memory function in adulthood
• Hippocampal-dependent memory impairments were prevented by co-consumption of AGE-breaker during early life
• Changes in the microbiome, specifically related to Lactococcus (genus), were directly correlated with contextual episodic memory performance, suggesting a functional link between AGEs, the microbiome, and neurocognitive performance
• Early life supplementation with Lactococcus probiotic showed potential to prevent AGE diet-induced memory impairments
Shifting to broader understanding of a “Western diet”(Intro)
• Western diet exposure during early life periods of development negatively affects neurocognitive development, notably hippocampal-dependent learning and memory.
Early life cafeteria-style Western diet model in rats Procedure
• Cafeteria diet (CAF, n=12) vs. standard chow diet (CTL, n=12)
o Male Sprague Dawley rats
o Beginning at postnatal day (PN) 26
o 30 d diet period before behavioral and metabolic testing
• CAF group switched to standard chow diet at PN 86, kept on diet for 30 d before testing
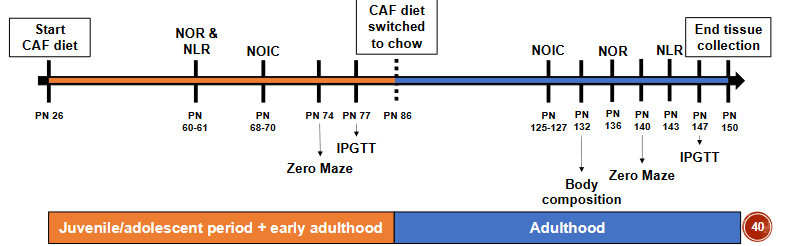
Body Weight Food intake: Minimal differences in metabolic outcomes, but change in hippocampal memories
Despite consuming more calories during early life, CAF rats showed no differences in body weight, glucose tolerance, or body composition compared to CTL rats.
Impairment in hippocampal-dependent memory persisted following 30 day healthy diet intervention.
A more healthy diet doesn’t fix adolescent Western diet symptoms.
Acetylcholine as a memory mediator(cholignergic innervation)
• The hippocampus receives dense cholinergic innervation from forebrain medial septum neurons
• The hippocampal cholinergic system is severely deteriorated in the brains of subjects with Alzheimer’s disease
Questions asked during AGEs experiment:
What are the impacts of early life consumption of a diet that has undergone the Maillard reaction to an extensive degree, resulting in a high level of AGEs, on neurocognition?
Can early life Western diet-induced memory impairments be reversed by a healthy diet later in life?
Does acetylcholine play a causal role in early life Western diet-induced memory impairments?
In vivo imaging of ACh release in the dorsal hippocampus with iAChSnFR and its results
Acetylcholine release is engaged in the discrimination of a novel object in context, and this phenomenon is absent in CAF(western cafeteria food) animals.
Acetylcholine release events in the hippocampus are predictive of memory performance.
Future research Considerations
• Consumption of a Western diet in early life leads to long-lasting memory impairments underscored by disruptions in acetylcholine signaling in the memory center of the brain
• Consumption of a diet high in advanced glycation end products in early life results in hippocampal-dependent memory impairments that appear to be related to the bacterial taxon Lactococcus lactis
• Future research is needed to understand further:
➢ What specific feature(s) of a Western diet are a detriment to cognition, and how their influence differ throughout the lifespan
➢ Food properties, especially related to food processing, that give rise to altered memory function and control of food intake
➢ How foods can be designed to impart positive effects on gut-brain health
Summary on Results of the study(Western DIET)
• Early life Western diet consumption resulted in memory impairments that persisted with a healthy diet intervention during adulthood
• Acetylcholine agonists administered into the dorsal hippocampus rescued Western diet- induced memory impairmentsdiet-induced, indicating a potential causal role of acetylcholine
• Western diet-induced hippocampal memory impairments were linked with disrupted hippocampus acetylcholine signaling
➢ Consumption of a high-fat, high-sugar Western diet in early life leads to long-lasting memory impairments underscored by disruptions in hippocampus acetylcholine signaling