AP chemistry-VSEPR MOLECULAR GEOMETRY
1/19
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Linear
A molecule where the atoms are in a straight line and is typically non-polar with a 180 degree bond angle.
-no lone pairs
-2 electron pairs
Lone pairs
Electrons dumped on the center atom that compresses bond angles
Electron pairs
All bonds count as one electron pair. (Lone pairs + pairs) or (dots+lines)
Trigonal Planar
A molecule with three atoms around the center atom and no lone pairs usually a non polar molecule.
sp2
3 electron pairs
0 lone pairs
120 degree bond angle
Bent/angular
A molecule with 3 electron pairs, one lone pair and two molecules on the center atom.
sp2
Polar
Less than 120 degree bond angle
3 electron pairs
1 lone pair
Tetrahedral
A molecule with 4 atoms around the center atom and 0 lone pairs.
4 electron pairs
0 lone pairs
Sp3
Nonpolar
109.5 degree bond angle
Trigonal pyramidal
A molecule with 3 atoms around the center atom and 1 lone pair
4 electron pairs
1 lone pair
Sp3
Polar
107 degree bond angle
Bent/angular
A molecule with 2 atoms on the central atom and 2 lone pairs
4 electron pairs
2 lone pairs
Sp3
Polar
104.5 degree bond angle
Trigonal bipyramidal
A molecule with 5 atoms around the central atom with 0 lone pairs
5 electron pairs
0 lone pairs
Sp3d
Non-polar
See-saw
A molecule with 4 atoms around the central atom and 1 lone pair.
5 electron pairs
1 lone pair
Sp3d
Polar
T-Shaped
A molecule with 3 atoms around the central molecule and 2 lone pairs.
5 electron pairs
2 lone pairs
Sp3d
Polar
Linear
A molecule with 2 atoms on the central atom and 3 lone pairs.
5 electron pairs
3 lone pairs
Sp3d
Non-polar
Octahedral
A molecule with 6 atoms on the central atom and 0 lone pairs.
6 electron pairs
0 lone pairs
Sp3d2
Nonpolar
Square pyramidal
A molecule with 5 atoms on the central atom and 1 lone pair..
6 electron pairs
1 lone pair
Sp3d2
Polar
Square planar
A molecule with 4 atoms on the central atom with 2 lone pairs
6 electron pairs
2 lone pairs
Sp3d2
Non-polar
T-shaped
Three atoms on the central atom with 3 lone pairs.
6 electron pairs
3 lone pairs
Sp3d2
Polar
Linear
A molecule with 2 atoms on the central atom and 4 lone pairs.
6 electron pairs
4 lone pairs
Sp3d2
Non-polar
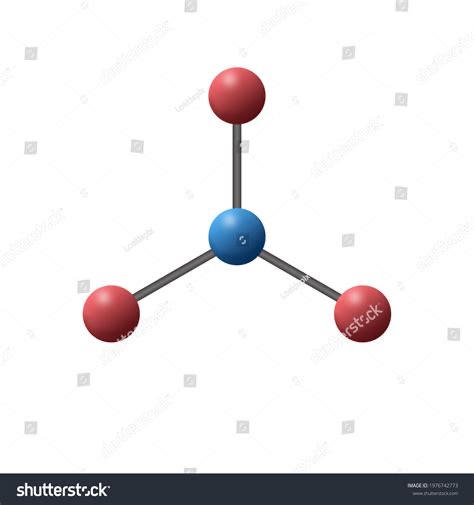
What shape is this?
Trigonal Planar
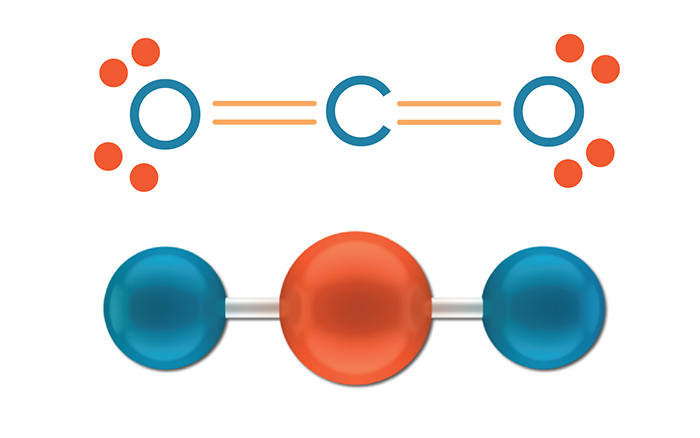
What shape is this?
Linear