Understand and be able to explain basic concepts : H, S, E, G, Q, W and how they relate to each other, and how they relate to P, V and T
H: enthalpy (heat content) Heat is the quantity of energy that floes across the boundary of a system in response to a difference in temperature between the system and its surroundings.
H= E+ PV
Qp( PV2+R2)_(PV1+E1)= H2-H1= Delta H
The enthalpy change in a reation is, therefore, the hea withdrawn from the surroudnins at constant pressure
If heat is given out during a reaction, it is said to be exothermic and H is negative. An endothermic reaction is one that takes heat from the surroundings and hence H is positive
H?
H: enthalpy (heat content) Heat is the quantity of energy that flows across the boundary of a system in response to a difference in temperature between the system and its surroundings.
H= E+ PV
Qp( PV2+R2)_(PV1+E1)= H2-H1= Delta H
The enthalpy change in a reaction is, therefore, the heat withdrawn from the surroundings at constant pressure.
If heat is given out during a reaction is, therefore, the heat withdrawn from the surroundings at constant pressure
If heat is given out during a reaction, is is said to be exothermic and H is negative. An endothermic reaction is one that takes heat from the surroundings and hence H is positive.
By convention, we assign an arbitrary “absolute” value of zero to the enthalpy of each of the elements in their standard stable form at 298.15k and a pressure of 10^5 Pa (1 bar)
CP
CP : the heat absorbed from the
surroundings at constant pressure (the
enthalpy) to a given temperature increment
W?
W: Work is the quantity of energy that crosses the boundary of a system and is convertible entirely into mechanical work in the surroundings, such as the lifting of weight.
Wexp= (force)x(distance)= (Pop x area) x (distance)
= Pop x (area x distance) = Pop DeltaV
Prescott Joule’s
If we consider the system plus the immediate surroundings, energy is conserved in all
processes. This fact was first clearly enunciated by the chemist, physicist, and physician Julius
Robert von Mayer in 1842 and eight years later was quantified in James
Prescott Joule’s classic experiment on the mechanical
Stability field:
Stability field: if some fundamental basic data can be
experimentally determined, we can calculate quantitatively
whether a certain assemblage of minerals (i.e., a rock), with or
without a fluid phase or melt, is stable at some particular
combination of P, T, and X
Effects of conditions change
Effects of conditions change: thermodynamic principles are
used to assess a geologic system or predict the effects that a
change in pressure (P), temperature (T), or composition (X)
may have on a stable rock/fluid/melt assemblage.
· What is a system? What are different states of a system?
A system is some portion of the universe that one might isolate (either physically or mentally) in order to study it. (e.g magma chamber, magma mantle, ect.)
The surroundings are the adjacent portions of the universe outside the system
Isolated system: A system that doesn’t exchange energy or materials with its surroundings
Closed system: A system that exchanges energy but not materials with its surroundings
Open system: A system that exchanges both materials and energy with its surroundings.
· What are the commonly referenced pressure and temperature for tabular thermodynamic properties.
Standard table form: 291.15K and pressure 10^5Pa
Second law of thermodynamics?
Kelvin-Planck Statement of second law of thermodynamics:
“its impossible to build a machine that can take heat from one place and turn it entirely into work without leaving some of the heat behind”
Another way of expressing the second law of thermodynamics:
For a real reaction to occur in an isolated system, entropy must increase. The reaction will continue until equilibrium is attained, at which point dS becomes zero and the entropy is a maximum.
Entropy: most easily perceived as randomness
Thinking of our own bedroom: it always tends to get messier; we always need to spend energy to clean and order it.
Third law of thermodynamics
The third law of thermodynamics states that the
entropy of a pure, perfectly crystalline substance is
zero at the absolute zero of temperature.
Combining the first and second law:
First law of thermodynamics
First law of thermodynamics: Can be captured in the following equation, which states that the energy of the universe is constant. Energy can be transferred from the system to its surroundings, or vice versa, but it can’t be created or destroyed.
A simple statement of the first law is that the total change in the energy of the system, E is given by the difference between the heat flow into the system and work done by the system
One of the thermodynamic properties of a system is its internal energy E, which is the sum of the kinetc and potential energies of the particles that form the system, Internal energy is proportional to temperature.
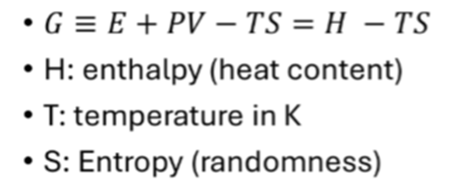
· How to write the Gibbs free energy change of a reaction?
Gibbs free energy (G) acts as a measure of the energy content of chemical systems; can be used to access the stability of chemical.
For a spontaneous reaction (irreversible) to occur at constant P and T, the value of G must be negative. Stable forms of a chemical system are those with the minimum possible Gibbs free energy for the given conditions.
For a spontaneous reaction (irreversible) to occur at
constant P and T, the value of G must be negative.
Stable forms of a chemical system are those with the
minimum possible Gibbs free energy for the given conditions
· How Gibbs free energy relates to the phase stability?
• All natural systems are governed by energy.
• Any macroscopic change in a system is accompanied by the conversion of energy from one form to another.
• If we consider the rock as the system...
• Lifting it: Potential energy increases
• Falling: Kinetic (heat lost to the surroundings) + mechanical energy (doing work to the ground)- unstable
• Landed: configuration of minimum energy of the rock (system) - Stable
• Not all natural systems change spontaneously to the minimum energy state
• Metastable: Some systems may exist in a state that is low in energy but not the lowest possible.
• There may be some energy barrier that must be overcome before the true
minimum energy state can be
At a constant T-P, ΔG controls the feasibility and direction of a reaction. Considering a reaction (crystallization, melting, degassing, metamorphism, etc.):
As T increases, which direction of the reaction S= L
should go?
As T increases, which direction of the reaction S= L
should go?
Why the slope for liquid is larger than solid?
As P increases, which direction of the reaction S= L should go?
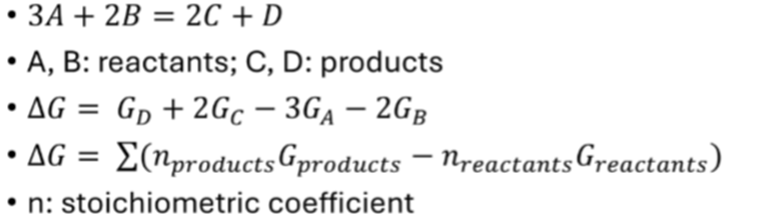
· How Gibbs free energy and entropy change relates to the direction of a reaction?
At a constant T-P, deltaG controls the feasibility and direction of a reaction.
Considering a reaction (crystallization, melting, degassing, metamorphism, ect)
If delta G is negative, then the products have a lower total free energy that the reactants (meaning that they are more stable), and the reaction should run from left to right, as written.
· How temperature and pressure change relate to the direction of pressure?
In general , pressure decreases with depth, and when pressure increases temperature also tends to increase due to compression
-in a closed system, increasing pressure can cause temperature to rise if the volume is constant
-in geological systems, increasing pressure with depth often leads to increase in temperature but the rate of change depends on heat conductivity, radioactive decay, and mantle convection
-when pressure decreases (such as upwelling mantle material), temperature may drop if heat isn’t added, leading to adiabatic cooling
· How solidus temperature normally changes as pressure increase? Why? Any special case?
The solidus temperature generally increases with pressure because:
-compression inhibits melting
-more energy is needed to overcome the stronger atomic bonding at higher pressures
Special cases:
1. Water-rich and volatile-rich systems
-in the presence of water or carbon dioxide the solidus temperature decreases with pressure
-this is because volatiles lower the melting point by breaking atomic bonds in minerals, facilitating melting at lower temperatures
-This explains why subduction zones experience melting at relatively lower temperatures-water released from the subducting slob lowers the mantle wedge’s solidus
2. Unusual mineral transitions
-some minerals might experience complex phase transitions that temporarily reduce or flatten the solid sop under certain pressure conditions
· What is the Le Chatelier’s principle? Give examples of how it applies to reactions.
If a change is imposed on a system at equilibrium, the position of the equilibrium will shift in a direction that tends to reduce that change.
Example:
1. Concentration change
· Example: In the reaction N2+3H2⇌2NH3N_2 + 3H_2 \rightleftharpoons 2NH_3N2+3H2⇌2NH3 (Haber Process for ammonia synthesis), if you increase the concentration of N2N_2N2 or H2H_2H2, the system will shift toward the right to produce more NH3NH_3NH3 to re-establish equilibrium.
· If you remove NH3NH_3NH3 as it's formed, the reaction will continue shifting right, increasing yield.
2. Temperature change
· Example: In the reaction 2SO2+O2⇌2SO32SO_2 + O_2 \rightleftharpoons 2SO_32SO2+O2⇌2SO3 (Contact Process for sulfuric acid production), which is exothermic, increasing temperature will shift equilibrium left, reducing SO3SO_3SO3 formation.
If the reaction were endothermic, increasing temperature would shift equilibrium right, favoring product formation.
3. Pressure changes (for gases)
· Example: In the reaction N2+3H2⇌2NH3N_2 + 3H_2 \rightleftharpoons 2NH_3N2+3H2⇌2NH3, increasing pressure favors the side with fewer moles of gas. Since the right side has 2 moles (NH₃) versus 4 moles (N₂ + H₂), increasing pressure shifts equilibrium right, producing more ammonia.
· Decreasing pressure does the opposite—it favors the side with more gas molecule
· How to change the melting point of a substance?
Melting points of a substance: Liquids can exist below Tm, unless some way to lower its G
One way to do it: Adding impurities!
Complex substances with more than one component (like rocks containing multiple minerals), state melting at a lower temperature than the melting point of its individual components.
Diopside melting
Crystallization of An-Di eutectic binary system
Lever rule
Ophitic texture
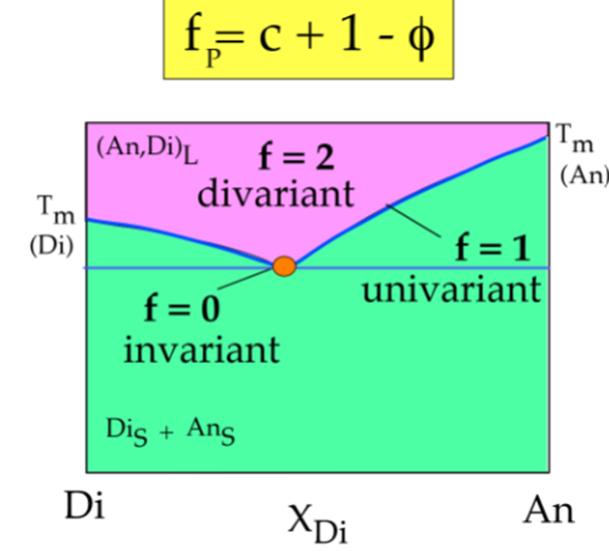
· What is the Phase rule? What does +2/+1 stand for? Under which conditions would one use +1 or +2 as the constant in that equation?
Gibbs phase rule
F= C- P + 1
F= # of degrees of freedom
P= # of phases
C= minimum # of components (chemical constituents that must be specified in order to define all phases)
1= 1 intensive parameters
Usually = temperature and pressure for use geologists.
Crystallization in eutectic systems
• Crystallization moves the liquid toward the eutectic composition
• Crystallization sequence (and related rock texture) depends on system starting composition.
• If all phases, including liquid, stay together (equilibrium crystallization) rock ends up with same composition as starting liquid (system) comp.
• If solid phases get removed (fractional crystallization) system composition moves toward eutectic.
· What are components? Degrees of Freedom? Invariant points?
Degrees of freedom: The number of intensive parameters that must be specified in order to completely determine the system
Phases: phases are mechanically separable constituents
Components: chemical constituents that must be specified in order to define all phases
Invariant points:
· What is an intensive variable? What are the common intensive variables in the phase diagrams we’ve worked with?
Intensive parameters: Usually = temperature and pressure for use geologists.
An intensive property is a bulk property, meaning that it is physical property of a system that does not depend on the system that does not depend on the system size or the amount of material in the system (extensive or extent of a system).
Examples of intensive properties include composition (X), temperature (T), pressure (P), refractive index (n) and density (p)
· What is an LLD? Liquidus? Solidus?, solvus?
LLD: Liquid line of descent: that evolutionary path that a magma follows as it cools and crystallizes
As minerals crystallize out of the melt, the remaining liquids composition changes
Liquidus: The liquidus us the temperature above which a material is completely molten
Below this temperature, solid phases begin to form during cooling
Solidus: The temperature below which a material is completely solid
Solvus: The solvus is the boundary that marks the limit of solubility of one phase in another.
Below this line, a single-phase solid solution exists, but crossing it (via cooling) causes another solid phase to precipitate out.
· Differences between Equilibrium crystallization and Fractional Crystallization
Crystallization moves the liquid toward the eutectic composition
Crystallization sequence (and related rock texture) depends on system starting composition
Equilibrium crystallization: If all phases, including liquid, stay together (equilibrium crystallization) rock ends up with same composition as starting liquid (system) comp.
Fractional crystallization: If solid phases get removed (fractional crystallization) system composition moves toward eutectic
· Types of melting? equilibrium vs fractional
Equilibrium melting: The opposite of equilibrium crystallization
Partial melting: The first melt of any mixture of Di and An must be the eutectic composition as well
Melt stays at that temp and composition until a phase is lost
Eutectic Melting:
First melt is at the Eutectic temperature and composition no matter what the solid composition is in the system
Invariant so systems stays at eutectic until one phase consumed and a degree of freedom is gained
Most primary magmas have a near-eutectic composition
Binary Peritectic Systems
Three phases enstatite = forsterite + SiO2
Peritectic reaction points: F=0
• Rounded/ resorbed olivine surrounded by orthopyroxene (OPX)
• Textural evidence of peritectic reaction
Peritectic reactions
Photomicrograph in XPL of olivine surrounded by (reacting with liquid to form) OPX
Incongruent Melting of Enstatite
F Melt of En does not → melt of same
composition
F Rather En →Fo + Liq i at the peritectic
Partial Melting of Fo + En (harzburgite) mantle
F En + Fo also → first liq = i
Solid Immiscibility and exsolution
• Solids can exolve (unmix) too.
• Get single exolved feldspar (perthite or antiperthite)
Perthite Exsolution
• Via solid-state diffusion some parts of the crystal get more Na-rich and other parts more K-rich forming exolution lamellae (shown).
• Solid diffusion is slow – exsolution only occurs in slowly cooled intrusives.
· What is a eutectic point? What is a peritectic point?
Eutectic point: The lowest possible point of the liquidus where all phases exist.
If the temp of the triple point is lower than the melting points of either of the two components, it’s called a eutectic point; but if it lies between the melting points of two components, it is called a peritetic point.
· What is the difference between the subsolvus and hyper solus feldspar systems? How could one tell the difference in thin section?
Subsolvus: Forms in lower temepratures and higher water pressure (hydrous conditions), has two feldspar phases (plag + K-feldspar), and slow cooling allows unmixing into twow feldspar types
Hypersolvus Feldspar system:
Forms under higher temps and lower water pressure (dry conditions). Has one feldspar phase (alkali feldspar). Faster cooling prevents full sepeartaion, forming perthitic texture
How to tell difference in thin section:
Subsovlus;
Disitinct plag and K-spar grains
Plag shows twinning
Kspar may have microcline twinning or perthitic texture
Hypersolvus system:
Large alkali feldspar crystals
Common perthitic texture (exsolutiin lamellae)
Lack of separate plag grains
· Know and be able to apply the phase rule.
Phases = 2 (Fo + Liq)
F = 3 - 2 + 1 = 2
If on liquidus, need to specify only 2 intensive variables to determine the system
X of pure Fo is fixed
Lever principle → relative proportions of liquid & Fo
C = 3: Ternary Systems:
Example 1: Ternary Eutectic
Di - An - Fo
Note three binary eutectics
No solid solutionNo solid solution
Green lines: cotectic linesGreen lines: cotectic lines
Ternary eutectic = M
Each side is the binary diagram
· Draw a liquid line of descent on a ternary phase diagram given a starting system composition.
· Be able to assess quantitatively a ternary eutectic diagram without solid solution.
· Be able to assess qualitatively a ternary peritectic systems and systems with one solid –solution pair.
Note: Binary character is usually maintained when a new component is added
-Eutectic behavior remains eutectic
-Peritectic behavior remains peritectic
-Solid solutions remain so as well
You should be able to assess chemical evolutionary trends on Harker diagrams relative to potential fractionating mineral phases (i.e. be able to draw a vector showing chemical evolution given a starting composition and the comp of fractionating or accumulating phases).
Assessing Chemical Evolutionary Trends on Harker Diagrams
1. What is a Harker Diagram?
A Harker diagram is a type of variation diagram that plots oxide concentration (SiO₂ vs. major oxides like FeO, MgO, CaO, Al₂O₃, etc.) to show how magma composition evolves during differentiation. These diagrams help identify fractional crystallization trends by revealing which minerals are being removed from the melt.
2. How to Assess Evolutionary Trends Relative to Fractionating Minerals?
· Fractional Crystallization removes minerals from the melt, shifting its composition away from the fractionating mineral.
· Accumulation of Minerals causes bulk composition to shift toward the mineral composition.
By looking at trends on a Harker diagram, we can determine which minerals were fractionating or accumulating.
Example of Fractionation Vectors:
· Olivine Fractionation (Removes Fe, Mg, Ni)
o SiO₂ increases
o MgO, FeO decrease
o Example: Basalt evolving toward andesite/dacite
o Vector Direction: Away from MgO-rich compositions
· Plagioclase Fractionation (Removes Ca, Al, Na, Sr)
o SiO₂ increases
o CaO, Al₂O₃ decrease
o Example: Andesite evolving toward rhyolite
o Vector Direction: Away from CaO, Al₂O₃-rich compositions
· Pyroxene Fractionation (Removes Fe, Mg, Ca)
o SiO₂ increases
o MgO, FeO, CaO decrease
o Example: Tholeiitic basalt differentiation
o Vector Direction: Away from FeO, MgO, CaO
· Amphibole Fractionation (Removes Mg, Fe, Ca, and H₂O-bearing components)
o SiO₂ increases
o FeO, MgO, CaO decrease
o Vector Direction: Away from amphibole-rich compositions
3. How to Draw a Chemical Evolution Vector?
1. Start with the Initial Magma Composition
o Example: Basaltic magma (low SiO₂, high MgO, CaO, FeO)
2. Determine the Fractionating Phase
o Example: If olivine is crystallizing, MgO and FeO decrease, and SiO₂ increases.
3. Draw the Vector
o Point away from the fractionating mineral's signature.
o Move toward the expected evolved composition (e.g., andesite, rhyolite).
Know that rocks are analyzed using spectroscopic techniques.
Spectrometer: Device for detecting and analyzing wavelengths of eletrmagnetuc radiation, commonly used for melcular spectroscopy; more boradly, any of various instruments in which an emission (as of electromagnetic radiation or particles) is spread out according to some property (as energy or mass) into a spectrum and measurements are made at points or regions along the spectrum. As used in traditional laboratory analysis, a spectrometer incueds a radiation source, and detection and analysis equipment.
Emission spectrometers excite molecules of a sample to higher energy states and alayze the radiation emitted when tey decay to the original energy state. Absorption sepctrometers pass radiation of known wavelength through a samlel, varying thr wavelegnths to produce a spectrum of results; the detector system reveas to what extent each wavelength is absorbed.
Example using X-ray Flourescence
Multiple elements are excited at one so then detect by using aone so then detect by using a spectrometer (a crystal latticespectrometer (a crystal lattice Braggs for WDS or SI-Braggs for WDS or SI- semiconductor for EDS) to semiconductor for EDS) to separate the different. Nonseparate the different. Non destructive, little to no chemicaldestructive, little to no chemical prep of sample, fast, multipleprep of sample, fast, multiple elements analyzed (good to aelements analyzed (good to a couple ppm for many elements couple ppm for many elements (even a bit lower)(even a bit lower)
Good for bulk samples
Non destructive, little to no chemical prep of sample, fast,
multiple elements anaylzed (good to a couple ppm for manymultiple elements anaylzed (good to a couple ppm for many
elements (even a bit lower)elements (even a bit lower)
Electron Microprobe
Bombard sample with an electron beam to excite sample
Can analyze small spot (micron) scan to make map
Good for mineral/glass analysis
To-go major elements in-situ analysis.
Mass spectrometry
Separate out charged isotopes (as ions) by their mass/charge ratio.
ll Measure ratios very accurately
How does this work?
A beam of charged particles are accelerated down a flight tube and around a curve by a potential flight tube and around a curve by a potential
difference (voltage). A magnet located at the curve deflects the path of the charged particles. The new path they follow depends on their momentum (mass) and how strongly they are affected by the magnet (charge). This splits the beam into several beams according to their mass-charge ratio
Different detectors are then aligned into the correct positions to detect the different beams
ICP-MS
Aspirate sample with Ar gas into a RF-driven plasma torch that turns sample into a plasma.
This is then introduced into some sort of spectrometer. These days most commonly a mass spectrometer. Sample introduced as fluid or via laser ablation. Excellent resolution, sensitivity. V. low detection limits.
Good for trace elements (to ppb levels)
Spot sizes 10-20 microns
Ideal for melt and fluid inclusions
o What are the typical abundance levels of a minor element? A trace element?
Major elements: Usually greater than 1%
SiO22 AlAl22OO33 FeO* MgO CaO NaFeO* MgO CaO Na22O KO K22O HO H22OO
Minor elements: Usually 0.1-1%
TiO22 MnO PMnO P22OO55 COCO2
Trace elements: Usually <0.1% or 1000 ppm
Everything else
·
· How to convert between weight% and atom%
Divide by Mol. Wt.
Must multiply by # of cations in oxide
Add all Atom prop. Then divide each by total = Atom %
· How to convert between FeO wt.%, Fe2O3 wt.% and calculate FeO*wt.%
FeO* =2*mwFeO*wtFe2O3/mwFe2O3 + mwFeO
· How is a mafic rock different in its major/minor element characteristics from a felsic rock?
Mafic rock is distinguished from a felsic rock by having a higher concentration of magnesium and iron, while felsic rock is richer in lighter elements like silicon, aluminum, sodium, and potassium.
· What is the SiO2 content of a basalt? Andesite? Dacite? Rhyolite?
Basalt 45-52% sio lowest silica content, Andesite 55-665% SiO2 intermediate silica, Dacite 63-69 SiO2 higher silica than andesite, Rhyoliite 65=75% sios high silica content
o What is a Harker variation diagram? What do they show?
Harker diagrams: These are variation diagrams in which the concentrations of an element or oxide are plotted (on the vertical axis) against those of sio2 (on the horizontal) for an igneous rock suite. If these plots show a clear trend or correlation (i.e all rocks fall on this trend), then all these rocks are probably related or comagmatic (i.e they define an igneous rock series)
SIO2 is a good chouce to plot against the concentrations of other elements and oxides since it shows a wide range of values
The previous figure shows the variation of major element oxides with silica in an igneous rock series. As the parental magma differentiates, SiO2, Na2O, and K2O increase, whereas MgO, FeO, TiO2 and CaO all decrease. Al2O3 on the other hand, slightly increases with differentiation and then decreases.
Liquid line of descent (LLD)
The chemical trend (or gradation in composition) exhibited by an igneous rock series if and when such a trend is interpreted to represent a change in the chemical composition of the parental magma of this series due to the process of fractional crystallization. A liquid line of descent is therefore equivalent to "the crystallization path" of the parental magma, and is best shown by plotting the chemical compositions of either aphyric (phenocryst free) or glassy volcanic rocks on variation diagrams.
Why should they idealy be aphyric or glassy?
* Care should be taken to avoid altered rocks
Harker diagram
Smooth trends - curvilinear
Model with 3 assumptions:
1 Rocks are related by FX
2 Trends = liquid line of descent
3 The basalt is the parent magma from which the others are derived
MOB Tholeiitic Suite
Little variation in SiO2 with differentiation
An example of a highly differentiated MORB suite
Using MgO on X-axis because SiO2 shows little variation
o How would you decide what oxide to plot on the X-axis?
Weight % oxide vs. MgO:
This is another type of variation diagram, where MgO is plotted on the horizontal axis instead of SiO2. This diagram is suitable for mafic rocks (e.g. basalts), because MgO can decrease considerably as basalts differentiate. On the other hand, SiO2 can show little change among the different types of basalts, making this oxide unsuitable for monitoring their differentiation.
SIO2 is a good chouce to plot against the concentrations of other elements and oxides since it shows a wide range of values
o What is an AFM diagram? Why is it useful? Can you draw the trends of different rock series?
The AFM diagram is a ternary plot in which the concentrations of Na2O + K2O (alkalis; A) FeO (F) and MgO (M) in an igneous rock are plotted after recalculation to a sum of 100%.
If the rocks plotted belong to a magmatic series, they will define a trend
Figure 8-2 also shows the difference between two commonly observed trends: an Fe-enrichment trend (representative of the differentiation of a tholeiitic magma; see below) and "a straight line trend"representative of the differentiation of a calcalkalic magma. This diagram can therefore be used as a tool for classifying different igneous rocks.
Bi-modal volcanism
Bimodal volcanism is normally explained as a result of partial melting of the crust creating rhyolitic magmas, during the emplacement of large volumes of relatively hot basaltic magma from a mantle source
Often associated with rifting or hotspot volcanism in continental or thick crustal environments
Fractional Crystallization Models in MORB Tholeiites
Showing slight decreases in SiO2 along LLD in MORB
Crystallization Modeling software:
MELTS, https://melts.ofm-research.org/
Petrolog, https://petrologsoftware.com/
° 37’ N Small OSC Geochemistry
Typical low-pressure liquid lines of descent calculated for the East
Pacific Rise = Fractional Crystallization
Note that more than one parent magma is needed to explain the variations.
E-Types (high K2O) also require a different enriched parent.
· What is the alumina saturation index? What does Peraluminous mean? What does Peralkaline mean?
Alumina Saturation Index (ASI) 🔥
The Alumina Saturation Index is a whole vibe in petrology — basically, it's a way to classify igneous rocks based on how much aluminum oxide (Al₂O₃) is in the mix compared to other major oxides.
It's calculated like this:
ASI=Al2O3(CaO+Na2O+K2O)ASI = \frac{Al_2O_3}{(CaO + Na_2O + K_2O)}ASI=(CaO+Na2O+K2O)Al2O3
👉 What that means:
Al₂O₃ = Alumina (aluminum oxide, queen of the crust)
CaO = Calcium oxide
Na₂O = Sodium oxide
K₂O = Potassium oxide
These other oxides represent the cations that would typically balance aluminum in minerals like feldspar.
What the ASI Number Says About the Rock:
ASI Value | Name | Vibes |
< 1 | Peralkaline | Giving spicy sodium & potassium overload — more alkalis than aluminum. Often found in volcanic rocks like phonolites and trachytes. |
= 1 | Metaluminous | Everything is balanced, babe — like the rock is at peace with itself (think diorites, granodiorites, or many basalts). |
> 1 | Peraluminous | Aluminum baddie alert — extra Al₂O₃ is left over after feldspars and feldspathoids form. This leftover aluminum usually gets snatched up by minerals like muscovite, garnet, or cordierite. |
o On major element variation diagrams – what do smooth curvilinear trend of data commonly represent? What about straight lines? What does an inflection point represent?
Smooth Curvilinear Trends 🔄
If the data points in a major element variation diagram follow a smooth, curving line, it usually means fractional crystallization is at play.
👉 Fractional crystallization is when minerals crystallize out of magma in a systematic order (thanks, Bowen’s Reaction Series!), causing the remaining melt to evolve compositionally over time.
As minerals like olivine, pyroxene, or feldspar start crystallizing and removing specific elements, the leftover magma gets more enriched in elements that aren’t being taken out, which creates that gradual, smooth curve.
🔥 Example:
MgO decreases smoothly as differentiation progresses because early-forming minerals (like olivine and pyroxene) suck up all the magnesium.
SiO₂ increases because those Mg-rich minerals crystallize first, leaving behind a more felsic melt.
Straight-Line Trends ➡
If the data points fall perfectly on a straight line, then you’re looking at mixing between two distinct magma sources or assimilation of crustal material into the magma.
This happens when you take two chemically different magma batches and blend them together, creating a linear interpolation between the two end-member compositions.
🔥 Example:
If a basaltic magma mixes with a more silicic magma (like rhyolite), you might see straight-line trends between major oxides like SiO₂ vs. MgO.
Inflection Points 🔄📍
An inflection point (where the curve suddenly changes direction or slope) usually means a major change in the dominant process affecting magma composition.
👉 What can cause an inflection point?
Switching mineral phases – If a different mineral starts crystallizing (e.g., plagioclase joins the party after olivine and pyroxene have been dominating), the element trends shift.
Magma mixing – If a new batch of magma is introduced mid-evolution, it can cause a break in the trend.
Assimilation of country rock – The magma eats up some of the surrounding crust, altering its chemical trajectory.
🔥 Example:
A major drop in Al₂O₃ when plagioclase starts crystallizing aggressively.
A shift in FeO/MgO trends when pyroxene replaces olivine as the dominant crystallizing phase.
✨ So, if you see a smooth curve – think fractional crystallization.
✨ If you see a straight line – think magma mixing.
✨ If there’s an inflection point – something big just changed in the system.
o You should be able to make some general conclusions about what a major element or trace element diagram shows or means including interpreting how magma chemistry will be affects by removal or accumulation of mineral phases.
· Given the chemistry of parent-daughter liquids and mineral phases on a bivariate plot you should be able to predict the phases being removed to evolve the magma chemistry.
o What is a “magmatic series”? What chemical parameters can be (and are) used to distinguish different series?
Magmatic Series and Petrogenesis:
Groups of rocks related in time and space
Temporal and spacial relationshios:
-mapping, superposition, radiometric dating, historical information
Chemical relationships:
-estimate Parental magma (lowest sio2, most mafic- high MgO)
-Test possibility for primary magma (in equilibrium with source, mantle?, lower crust?)
-Evaluate if more evolved (higher sios or lower MgO) derivative rocks can be related by igneous processes (Fractional crystallization, partial melting, magma mixing)
Igneous rock series
Igenous rock series: Is a group of igneous rocks that are genetically related (co-magmatic) and which were collected from a relatively small sarea (filed area, volcano; pluton). Rocks of an igneous rock series therefore commonly display a continuous graduation in chemical composition from the most madic to the most felsic in the group.
Igneous rock suite:
Igneous rock suite: Is a group of igneous rocks collected from an igneous province. These rocks may or may not be genetically related
Igneous province
Igneous province: A field area where igneous activity has occurred over a distinct period. Often associated with plate tectonic feature.
o Into what two series can the alkaline series be separated?
· Subalkaline vs Alkaline
Variation Diagrams: How do we display chemical data in a meaningful way?
Like to see how chemical parameters co-vary with one another...provides better constraints to figure out reason for observed data.
Bivariate Plots (Harker Diagrams)- 2 parameters, Classically SiO2 vs other oxides but MgO is also common
Can stack bivariate plots w/ common x-axis to see more parameters at once
Ternary plots- 3 parameters (sometimes more enlightening)
Spider diagrams (normalized- compared to a reference)
many elements represented (typically trace elements)
Ol formula
(Mg, Fe)2SiO4
enstatite
Mg2Si2O6
Diopside
CaMgSi2O6
Amphibole:
Hornblende: Ca2(Mg,Fe)5Si8O22(OH)2
Also Na-rich amphibole
These are hydrous minerals
Dark minerals with Mg, Fe
Biotite: K
Biotite
K(Mg,Fe)3AlSi3O10(F,OH)2
These are hydrous minerals
Dark minerals with Mg, Fe
Biotite: K
Anorthite
CaAl2Si2O8
Albite
NaAlSi3O8
Othoclase
KAlSi3O8
Muscovite
Muscovite: Al, K rich silicates hydrous
· What are the two magma series within the subalkaline group of rocks/magmas
AMF diagram can further subdivide the subalkaline magma series into a toleiitic and a calc-alkaline series
o Chemically, how does the tholeiitic series differ from both the alkaline and calcalkaline series. Why does it differ?
Tholeiitic suites are subalkalic and show distinct iron enrichment trends
Calcalkaline suites are transitional to alkaline suites and have no iron enrichment trends
TH: Fe enriched-depleted trend
CA: Fe depletion trend
The salient feature in many TH-CA discrimination diagrams is enrichment in Fe during magma evolution
Peacock Index:
Calcalkaline = 56-61 %SiO2
Alkaline < 51
The total alkali - silica diagram:
This plot, which is useful for the classification of volcanic rocks
(see chapter on classification), is also useful for distinguishing
between two types of parental magmas (or of magmatic rock
series or trends): alkalic and tholeiitic (Fig. 8-11). Alkalic
magmas are produced by partial melting at considerable depths
and differentiate into a specific group of rocks, with the most
differentiated ones rarely, if ever, becoming SiO2-oversaturated.
Tholeiitic magmas form at shallower depths and may
differentiate to the SiO2-oversaturated rhyolites.
What do silica saturation and silica undersaturation mean? Which series is typically oversaturated? Undersaturated
Undersaturated: Alkaline series
Oversaturated: subalkaline series
Classification of Volcanic Rocks Geochemical
Mass Balance Equation For Fractional Crystallization
3 phase crystallization and LLD vectors
3 phase crystallization and LLD vectors
Figure 6e illustrates the effect of extracting a solid solution, or two minerals in which the ratio varies continuously (as would occur with a curved cotectic). In this
case, the bulk mineral extract moves along the line from B toward A. As it does so, the melt composition must move directly away from a shifting bulk extract point, resulting in a curved liquid line of descent, similar to those for Al2O3, MgO, and Na2O in
Figure 2