Introduction to the periodic table and the trends
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
Atoms

•are the simplest and smallest particle composed of protons, electrons, and neutrons.
proton

•is a positively charged particle inside the nucleus.
neutron

is a neutral particle also inside the nucleus
electron

is a negatively charged particle that orbits the nucleus.

What is the PERIODIC TABLE?
oShows all known elements in the universe.
o
Organizes the elements by chemical properties

What is the ATOMIC NUMBER

oThe number of protons found in the nucleus of an atom

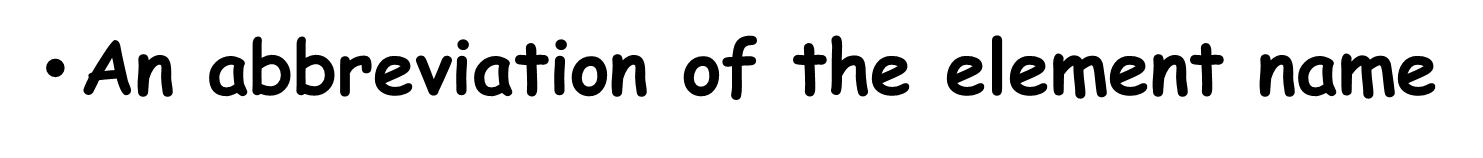
What is the SYMBOL

An abbreviation of the element name
What is the ATOMIC WEIGHT
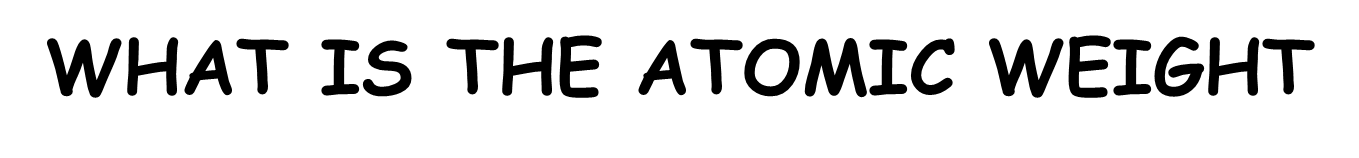
The number of protons and neutrons in the nucleus of an atom

# of PROTONS

ATOMIC NUMBER

# of ELECTRONS

ATOMIC NUMBER

# of NEUTRONS

ATOMIC WEIGHT - ATOMIC NUMBER

Solid

§ Particles held tightly
§ Very close together
§ Regular arrangement
§ Vibrate
§ Can’t move from place to place

Liquid
§Particles held weakly
§ Very close together
§ Random arrangement
§ Vibrate
§ Constantly move past each other

Gas
§No attraction between particles
§ Far apart
§ Random arrangement
§ Vibrate
Move quickly i

Chemical Property -

a property used to characterize materials in reactions that change their identity. Ex: burning something
Physical Property


- a characteristic of a substance that can be observed without changing the substance into something else. Ex: measuring something’s length, color, mass or volume
Metals
are lustrous, malleable, and are good conductors of heat and electricity

Non-metals
•are elements that do not share the properties of metals.
Metalloids

are elements that share some, but not all the properties of metals
Lustrous

•means shiny or reflective of light.
–Coins and jewelry are shiny and reflective .

Malleable

•means capable of being shaped.
Aluminum foil is shaped or molded around food items to keep them fresh
Good Conductor

•means being able to allow electricity and heat to flow through.
−When you think about the wires we use for
electrical devices, they are mostly made of
copper and other metals.

Alkali Metals
•Elements in Group 1 (not including Hydrogen).
•Very reactive metals. Always combine with something else in nature.
•Salt – an Alkali Metal,
Sodium, and another
element, Chlorine,
combined.

Alkaline Earth Metals

•Elements in Group 2.
•Reactive Metals that are always combined with non-metals in nature.
Several of these elements are important mineral nutrients, like Calcium
Transition Metals

•Elements in Groups 3-12.
•Less reactive, harder metals.
Includes metals used in jewelry, money and construction

Boron Family
•Elements in Group 13.
•Boron has properties of both metals and non-metals.
•The rest of the elements in this group are metals.

Carbon Family
Nitrogen Family
•Elements in Group 14.
•Contains elements important to life and computers.
•Carbon is the basic element in all organic compounds.
•Silicon and
Germanium are
important
semiconductors.

Nitrogen Family
•Elements in Group 15.
•Nitrogen makes up more than ¾ of our atmosphere.
The red tip of matches is made of phosphorous
Oxygen Family or Chalcogens

•Elements in Group 16.
•Oxygen is necessary for respiration.
Many things that have a bad odor contain sulfur

Halogens
•Elements in Group 17.
•Very reactive, diatomic non-metals.
•Always found combined with other
elements in nature.
•Chlorine is used to keep bacteria out of swimming pools.
Noble Gases
•Elements in Group 18.
•VERY reactive gases.
•Used in lighted neon signs.
Helium is used to make party balloons float

What is an ELEMENT?
oA substance composed of a single kind of atom.
o
oCannot be broken down into another substance by chemical or physical means.

What is a COMPOUND
A substance in which two or more different elements are CHEMICALLY bonded together

Atomic Radius

Since a cloud’s edge is difficult to define, scientists use define covalent radius, or half the distance between the nuclei of 2 bonded atoms.
Atomic radii are usually measured in picometers (pm) or angstroms (Å). An angstrom is 1 x 10-10 m.

Covalent Radius

Two Br atoms bonded together are 2.86 angstroms apart. So, the radius of each atom is 1.43 Å.

Atomic Radius

The trend for atomic radius in a vertical column is to go from smaller at the top to larger at the bottom of the family

Effective Nuclear Charge

Effective nuclear charge is the pull that an electron “feels” from the nucleus.
The closer an electron is to the nucleus, the more pull it feels
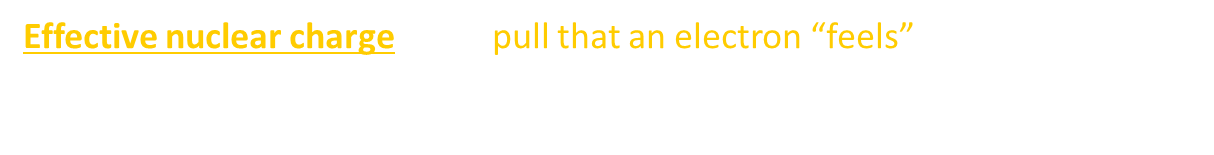
Ionization Energy

This is the second important periodic trend.
If an electron is given enough energy to overcome the effective nuclear charge holding the electron in the cloud, it can leave the atom completely.
The atom has been “ionized” or charged.
The number of protons and electrons is no longer equal.

Electronegativity

Electronegativity is a measure of an atom’s attraction for another atom’s electrons.
It is an arbitrary scale that ranges from 0 to 4.
Generally, metals are electron donators and have low electronegativities.
Nonmetals are electron takers and have high electronegativities.
What about the noble gases?
Do they need anyelectrons?

Overall Reactivity
This ties all the previous trends together in one package.
However, we must treat metals and nonmetals separately, as one will gain electrons and one will lose electrons.
The most reactive metals are the largest since they are the best electron givers.
The most reactive nonmetals are the smallest ones, the best electron takers.
