KBKF15 - WEEK 6 - PROTEIN TURNOVER, AA CATABOLISM & AA BIOSYNTHESIS
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
What does “Protein turnover” means?
All proteins has a lifespan, when that is over, the proteins has to be replaced by new proteins.
degradation and resynthesis happens constantly.
Proteins has variating half-lifes.
Defective proteins are degraded.
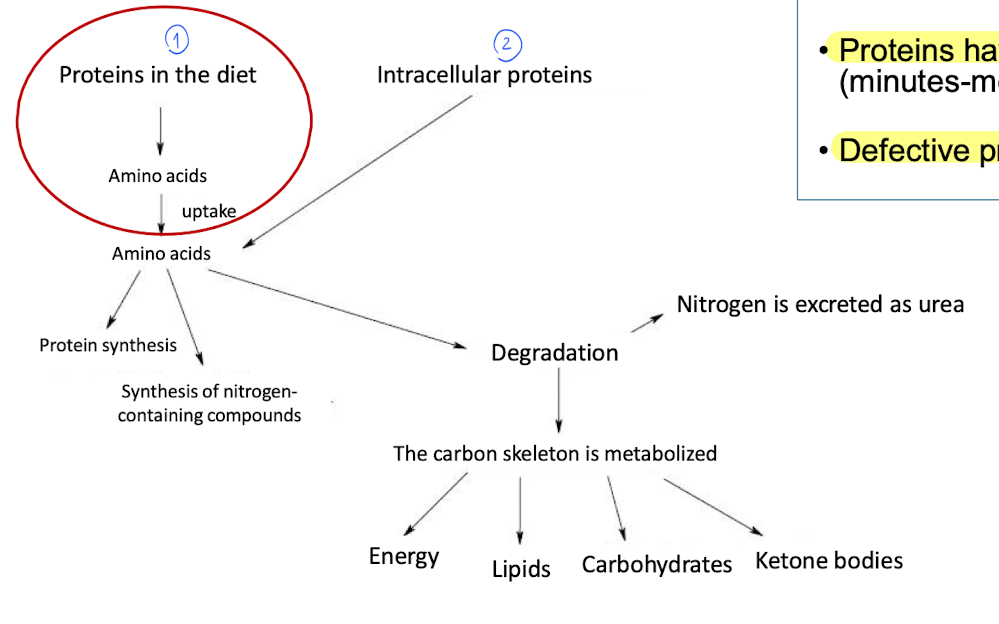
We have 2 sources of proteins
Diet
Intracellular protein
Ubiquitin and Degron - generelt
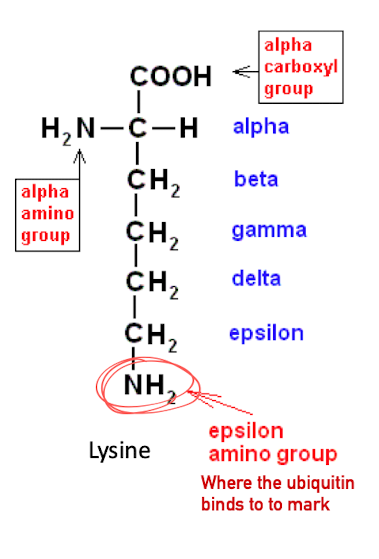
Degron : A specific AA-sequence which indicates that the protein should be degraded. Beroende på vilka AA som finns i N-terminalen blir det olika långt lifespan.
Ubiquitin binds to the target protein when the lifespan is ending. it will bind to the epsilon amino group in Lysine (AA) in the N-terminal (?) of the intracellular protein.
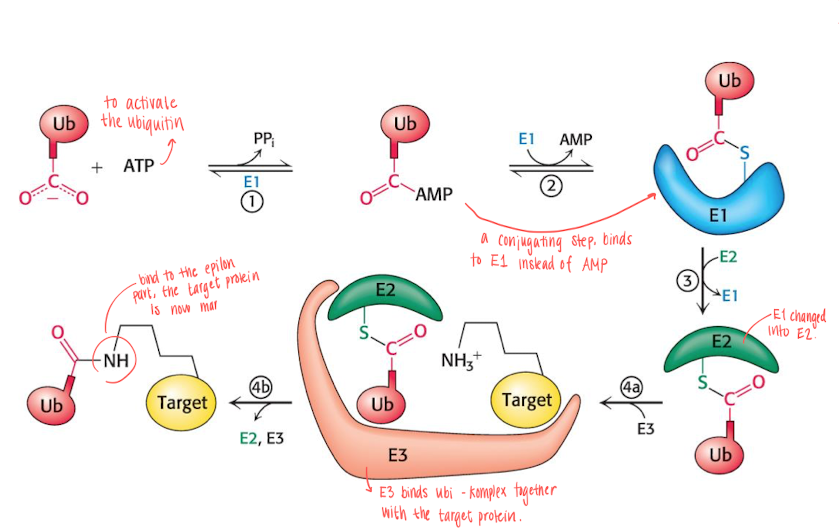
How does ubiquitin bind to the target protein?
the process requires 3 enzymes: E1 (Ubiquitin activating enzyme) , E2 (Ubiquitin conjugating enzyme) , E3 (Ubiquitin protein ligase)
4 or more ubiquitin needs to be bond for degradation, since ubiquitin is also utilized in other processes.
ATP is used to activate the ubiquitin (ATP —> AMP).
The AMP on the ubiquitin is substituted with E1, which is later substituted with E2.
the E2 complex will travel to the E3 complex, which the target protein also does.
The E3 complex will bind the ubiquitin to the protein complex’s epsilon amino group, and the protein will thus be marked.
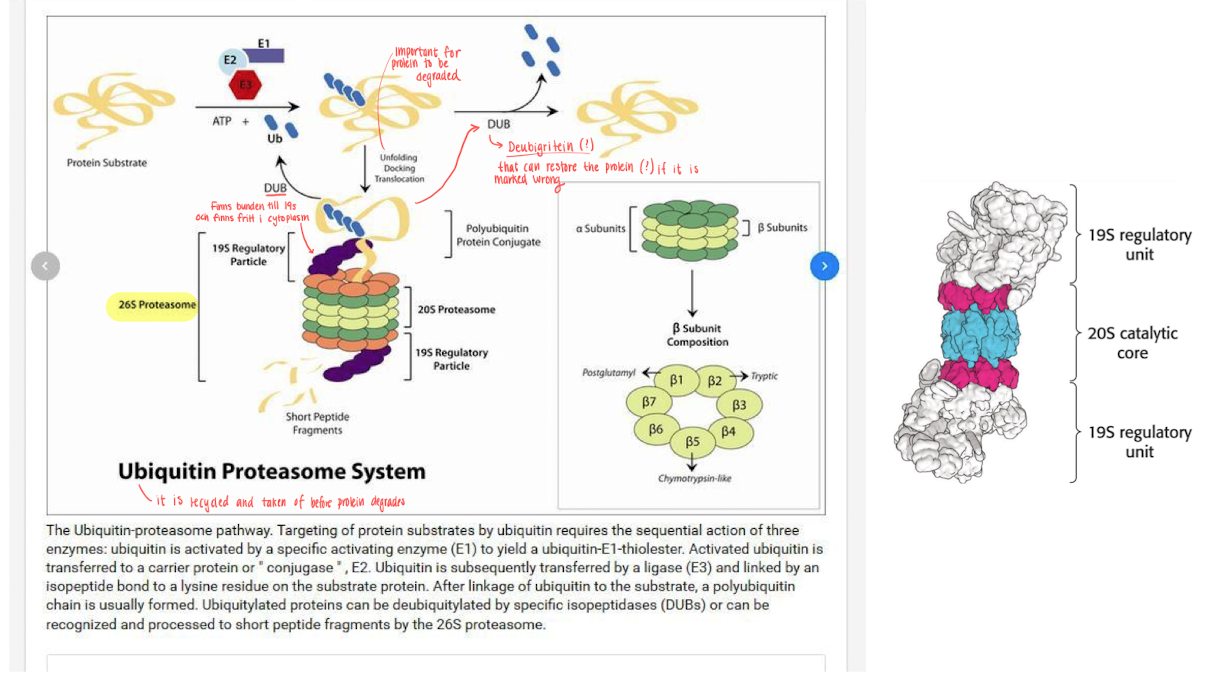
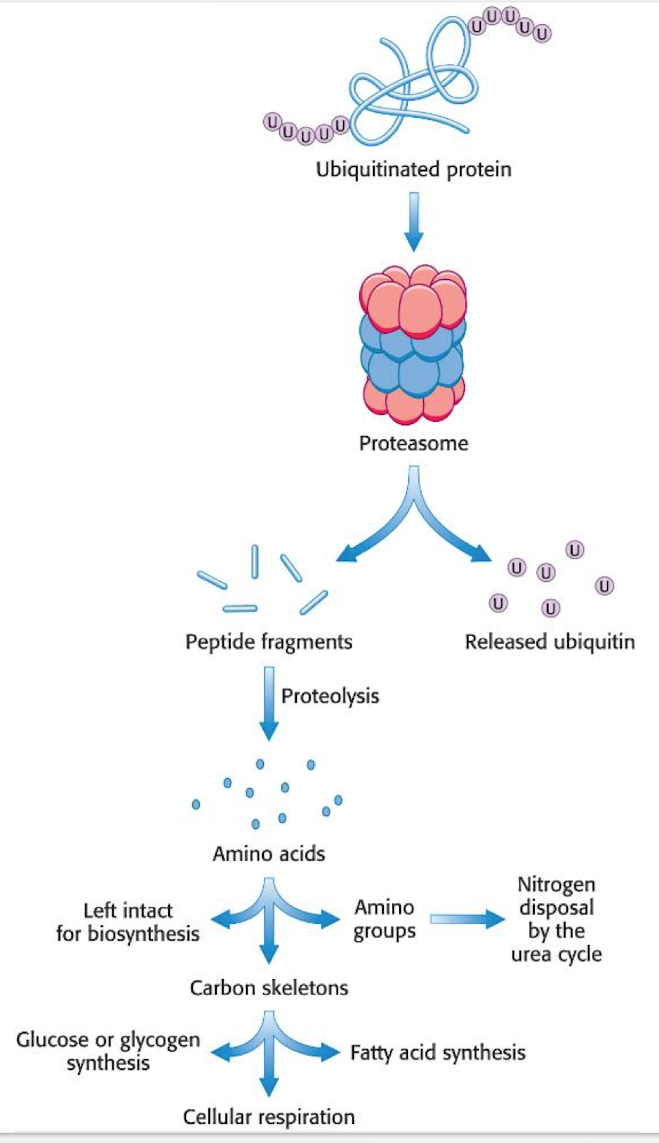
Protein degraded by - “The executer” - 26S proteasome.
the proteasome has 2 parts
19S - the regulatory part, which binds specifically to ubiquitin and cleaves it of from the protein to recycle. It then unfolds the protein and delivers it to the 20S.
20S - Catalyses breakdown, formed like a barrel and has 3 active sites, small peptides are realesed as the protein is broken down.
Protein degradation - what does this regulate?
regulates biological functions
ubiquitin is reused.
Free AA are either broken down or used in new protein synthesis.
protein degradation regulates: transcription, organ formation, inflammatory response etc.
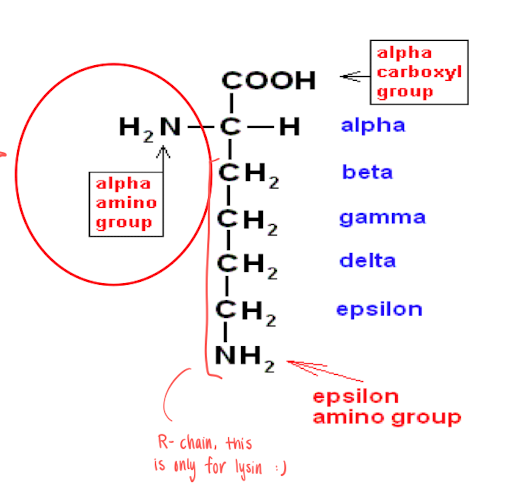
Nitrogen removal in AA
In the N-terminal
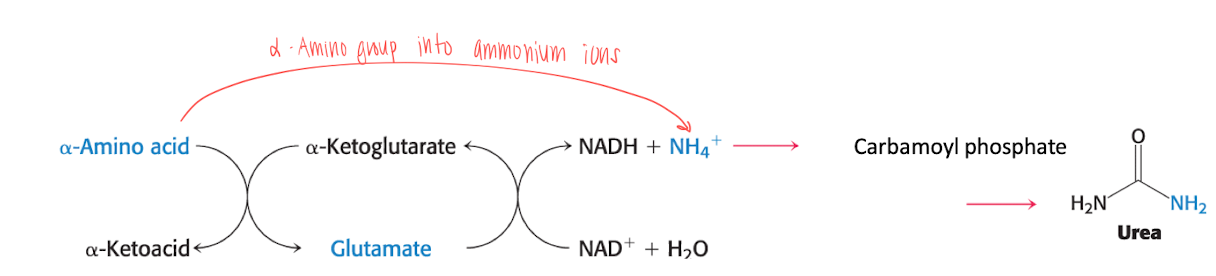
Alpha amino group is turned into ammonium ions. Which is then turned into urea (excreded through kidney)
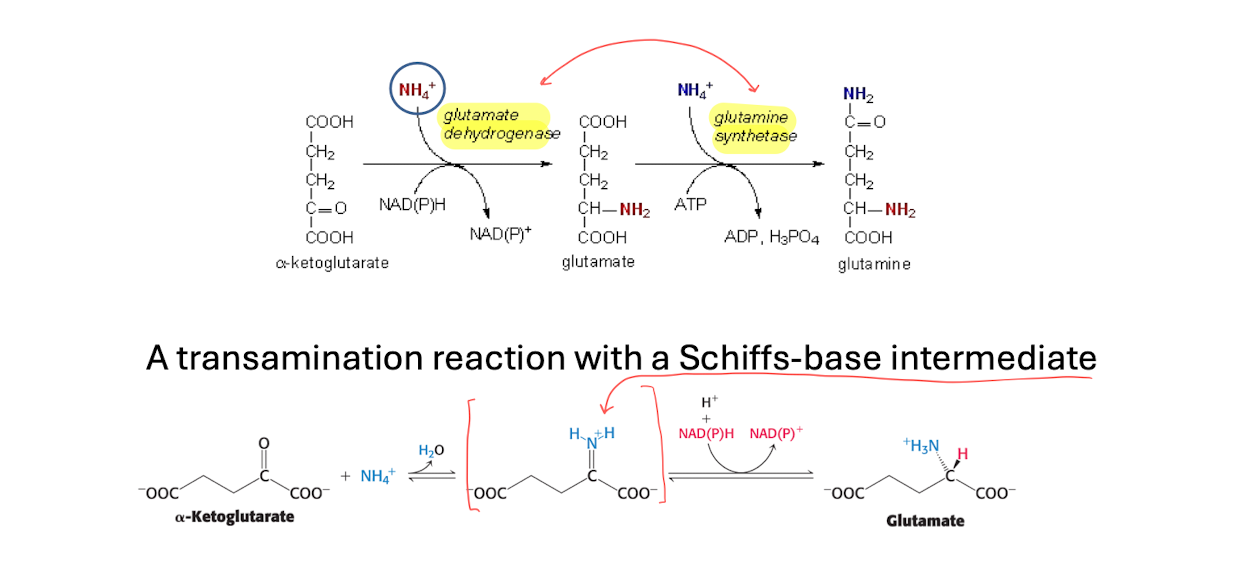
transamination
Glutamate is formed when alpha-amino-acid is turned into alpha-ketoglutarate.
Glutamate is then oxidized to form NH4+ and alpha-ketoglutarate and (NADH+ + H+) using NAD+
NH4+ is turned into Carbonyl phosphate, which goes into the urea cycle to form urea, which is then excreted through the kidneys.
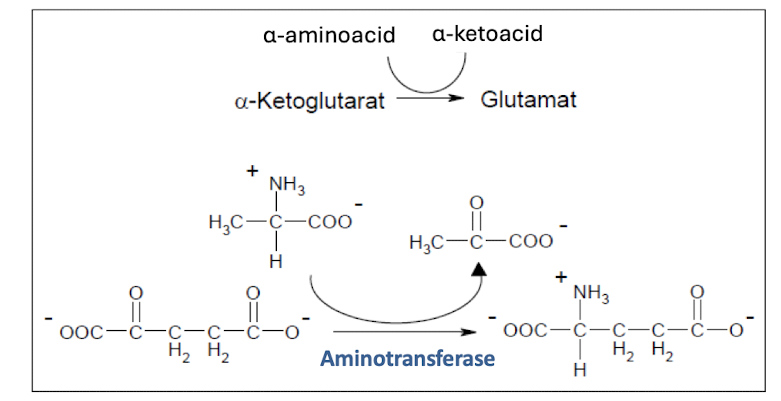
More of Transamination
It forms free NH4+ cells, which can be harmful
Amino-transferases uses PLP as prosthetic group, which forms a schiff-base intermidiate with aminotransferases. *
(Undantag) Ser and Thr can be converted into NH4+ directly.
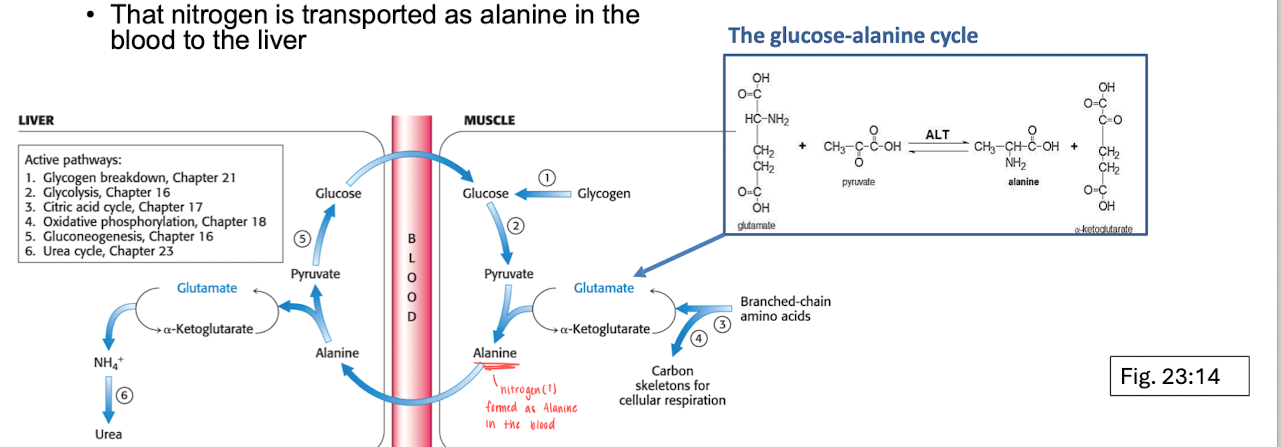
Nitrogen transport - Urea cycle
Urea cycle occurs in the liver, nitrogen is transported as alanine in the blood into the liver. Alanine (which is a AA) then acts as the alpha-AA to start the urea formation.
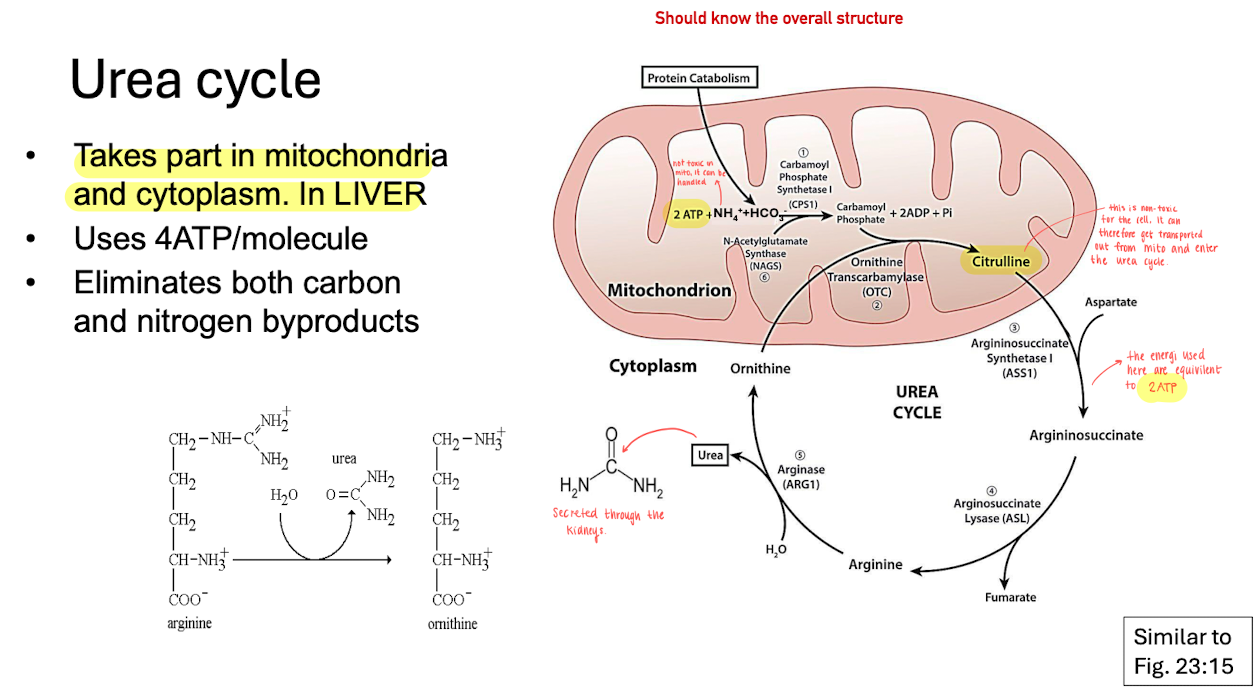
Urea cycle
Takes place in the mitocondria matrix & cytoplasm in the liver cells.
Uses 4 ATP / molecule
Eliminates carbon and nitrogen byproducts.
the urea cycle is lined to the gluconeogensis process (where glucose is produced).
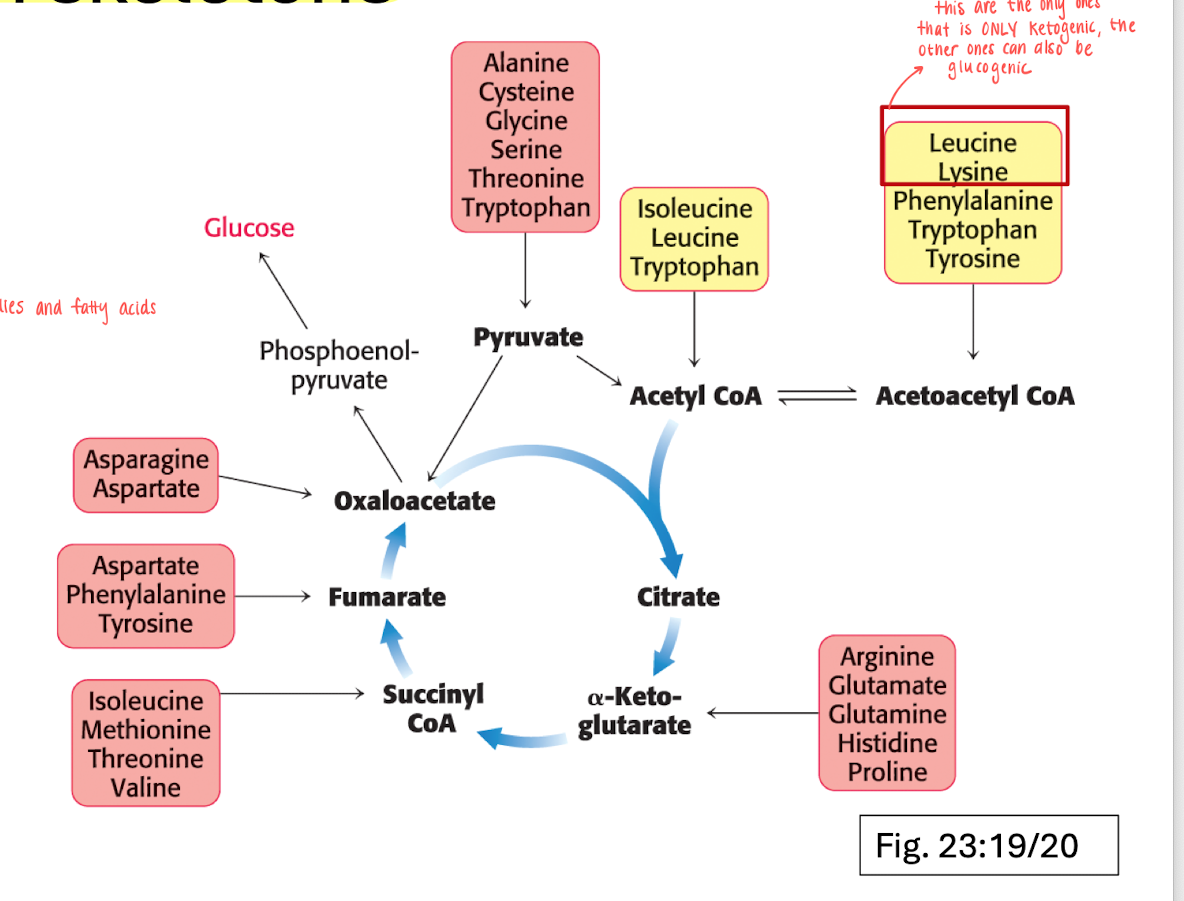
AA carbon skeleton structures
Carbon skeletons are funneled into 7 molecules.
AAs are either ketogenic (can form ketobodies and FA) or glucogenic (goes into the TCA-cycle)
Leucine and Lysine are the only AAs that is fully ketogenic, the rest of the moelcules can also be glucogenic.
Biochemical proteins with biosynthesis of AAs
Nitrogen fixation (N2 is very inert)
stereochemical control of AA
Proper concnetration of AA inside the cells.
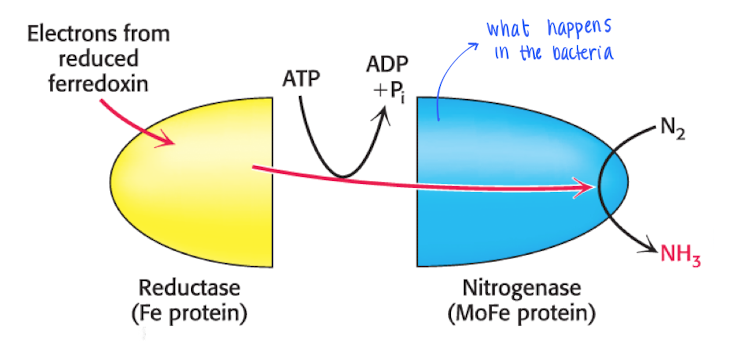
Nitrogen fixation
Bacterias living in synbios with plants fixates the nitrogen gas using 16ATP, 8H+ and 8e-.
The process is catalyzed by (Fe-S protein) and the e- comes from reduced ferrodoxin, 2e- used in each step ofthe process.
N2 —> 2NH3
Nitrogen can also be fixated in other ways, t.ex Abiotic
Through lighting and UV produces NOx
Industrial processes e.g Habor Bosch process. However, these processes requires a lot of energy.
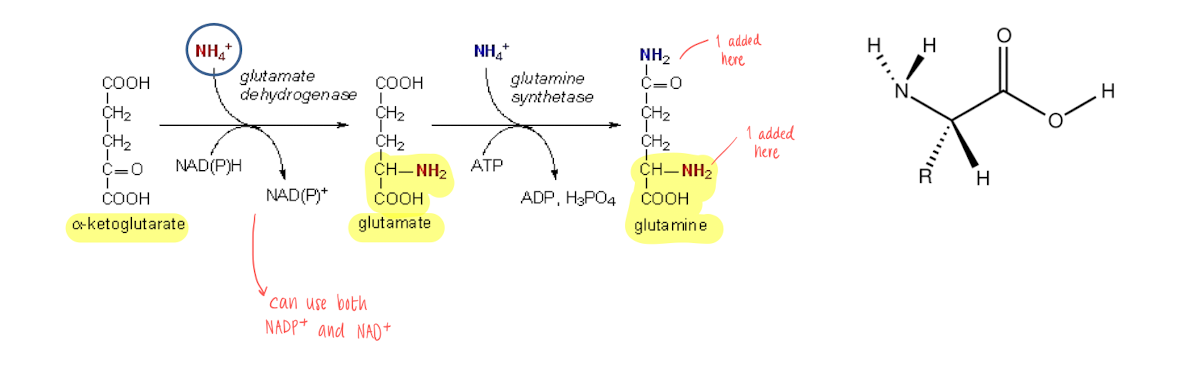
NH4+ - How are they put into AAs?
Glutamate and glutamine : nitrogen donors, the reactions needs NADH+/NADPH+ and ATP.
Glutamate : alpha amino group
Glutamine : side chain amino group
Stereochemical control of AA
Alla AA förutom glycin är kirala. vilket innebär att de har ett stereocenter (C) som är kopplade till 4 olika sidokedjor. Detta innebär att det är viktigt att den rätta isoformen är presenterad.
stereocenters occurs when putting in nitrogen in AA, and in schiffs-base intermediates during transamination.
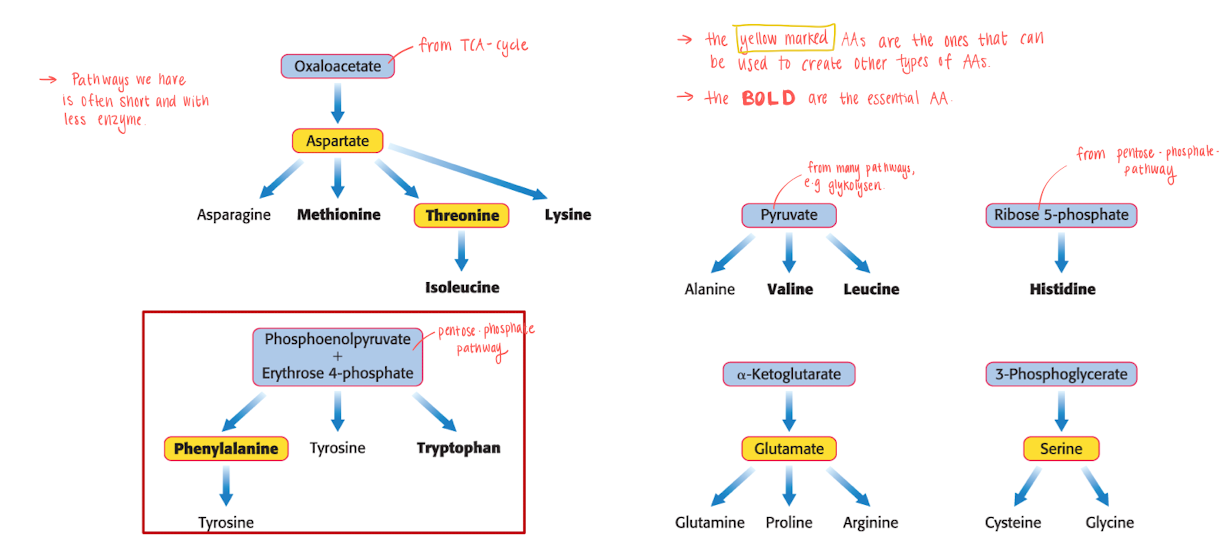
AAs are made form intermediates of the KREB cycle and other pathways
The yellow marked AAs that can be used to create other types of AAs.
the BOLD ones are essential AAs. Non essential AAs typically has more simple biosynthetic pathways.
Shikimic acid pathway
We don’t have this pathway (animals) since we lack ESPS-syntase
Shikimate and Chorismate are intermediate in aromatic amino acids. Pathway inhibited by glyphosate.
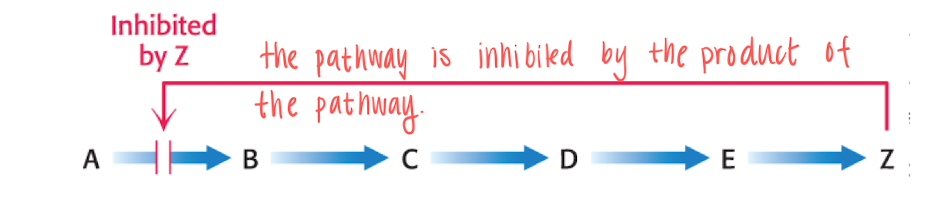
How is AA synthesis regulated?
It is regulated by feedback regulation of the end product of the pathway. the end product will inhibit the committed step (which is the first irreversible step at a branching point of a pathway).
Since the pathway is branched, we have to control several products, this is solved by Feedback inhibition + inactivation.
T.ex if we have a lot of Valine and low Isoleucine —> activate the production of isoleucine. (feedback)
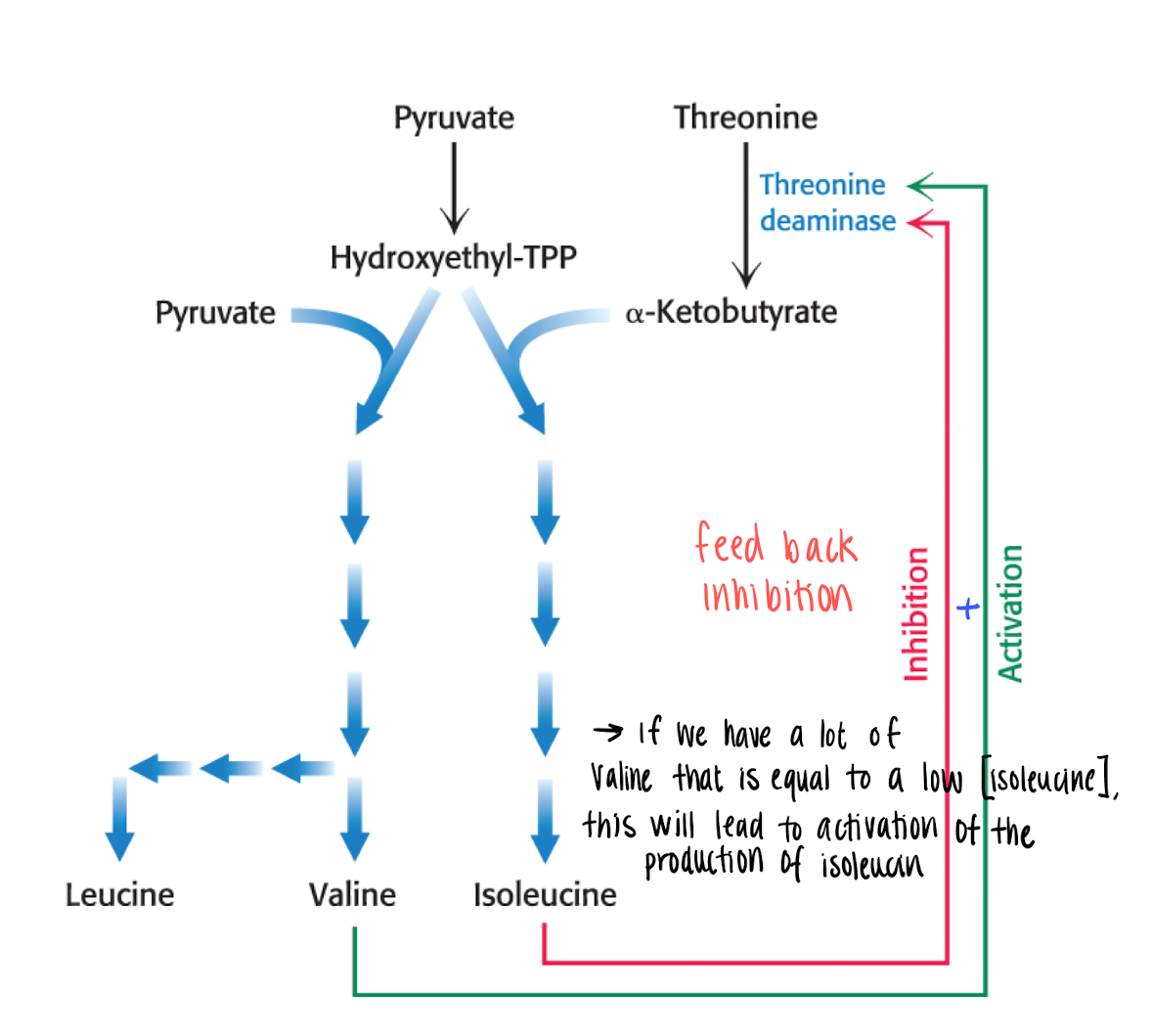
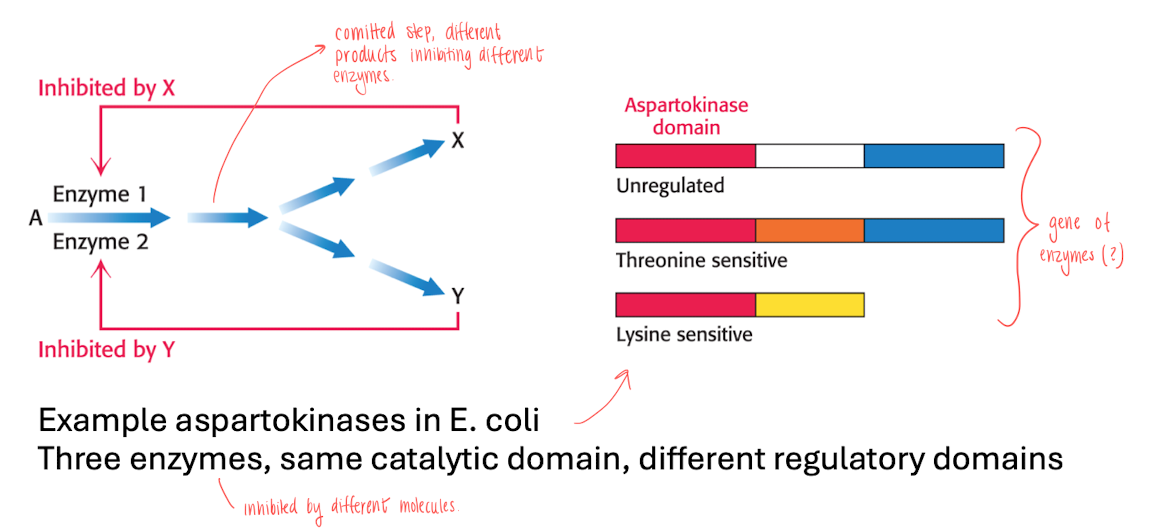
Enzyme multiplicity in a committed step of a pathway
committed step (irreversible at branching point in pathway), catalyzed by more than 1 enzyme, therefore the reaction needs to be inhibited with multiple molecules.
T.ex aspartokinases in e-coli, regulated by 3 enzymes, which has the same catalytic domain but different regulatory domains.
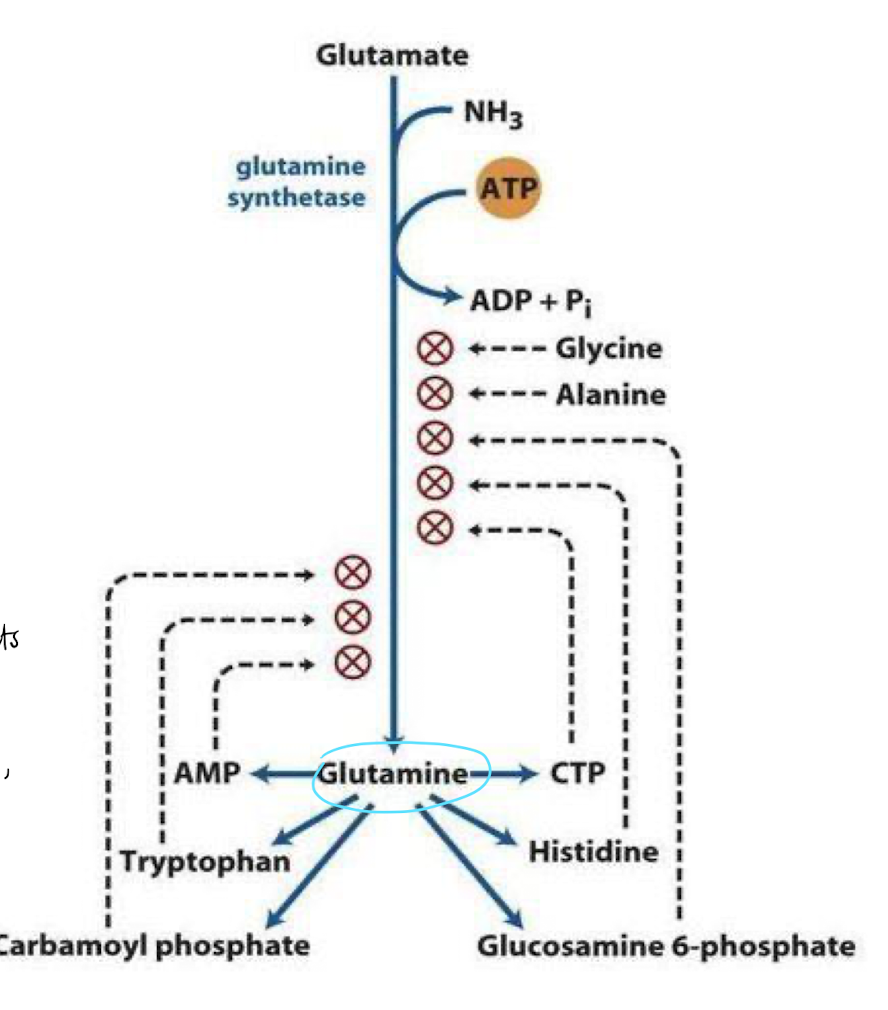
cumulative feedback inhibition
when the pathway is inhibited by multiple end products that each acts independently.
T.ex glutamine (the glutamine synthetase) pathway, each inhibitors acts independently, each decreasing a fixed percentage of the activity level.
AAs as metabolic precursors (intermediates that are oxidized to generate ATP or used to synthesize macromolecules)
Expected to know the basics
Nucleotide bases: Purines, pyrimidines
Signaling molecules: NO, hormones, neurotransmitters (immune and nerve systeme)
Heme group: Cytochromes, chlorophylls, hemoglobins