Electron Donating and Withdrawing groups
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
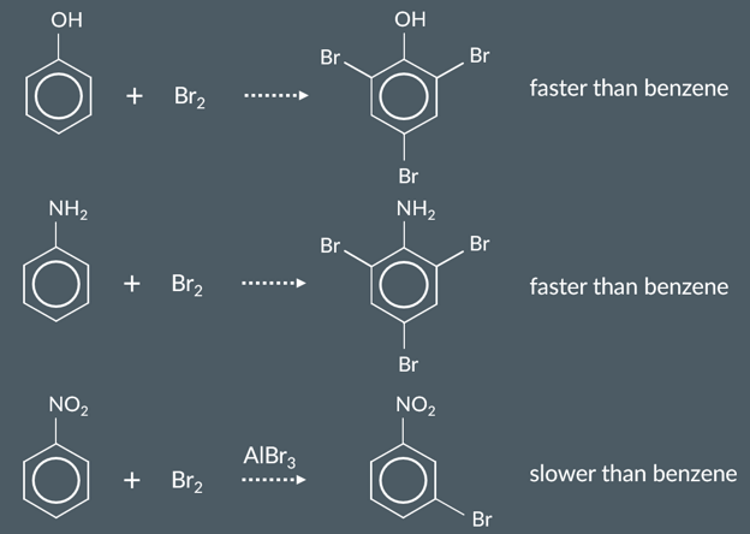
These observations suggest that bromine reacts…
more readily with phenol than with benzene.
more readily with phenylamine than with benzene.
less readily with nitrobenzene than with benzene.
Phenol has more electrons in its pi system than benzene.
This means that phenol’s pi system has…
greater electron density than benzene’s pi system.
Electrophiles react more readily with phenol and phenylamine than with benzene because.
A lone pair of electrons is delocalised into their pi systems.
their pi systems have greater electron density.
electrophiles are more attracted to them.
We call OH and NH2…
electron donating groups
OH and NH2 are what type of directing groups?
2,4 directing groups
Electrophiles react less readily with nitrobenzene than benzene.
Based on what we’ve seen, we’d predict that this is because NO2…
withdraws electrons from the pi system.
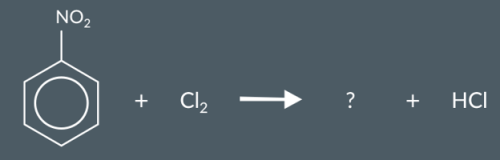
Which product are we most likely to form in this reaction?
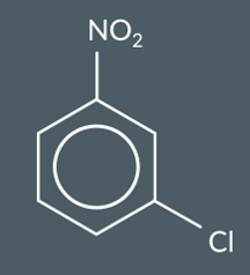
NO2 is a ————— group and a —— directing group
1) electron withdrawing
2)3
Predict the major product of this reaction.
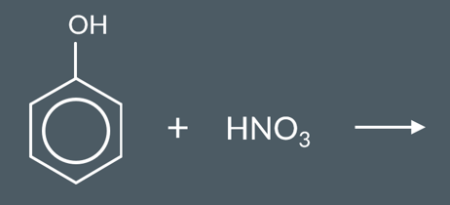
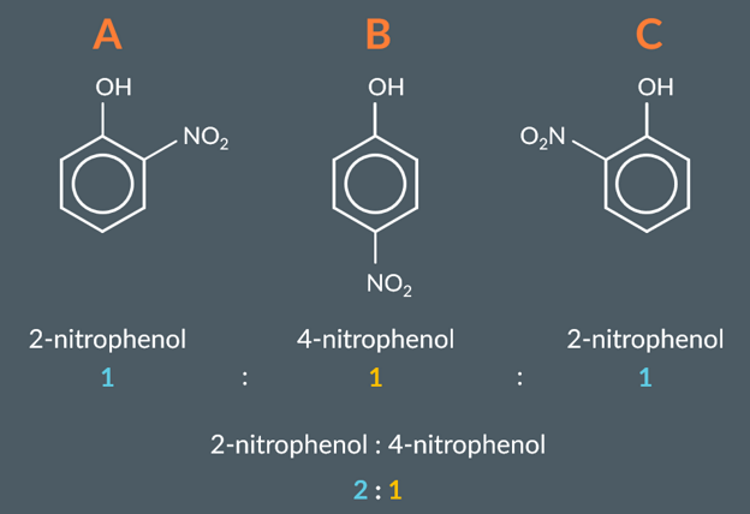
The OH group in phenol is a 2,4-directing group, so we expect the NO2 group to substitute at the 2nd, 4th or 6th carbons.
If NO2 substitutes at these three positions evenly, it would produce equal amounts of compounds A, B and C (below).
The 2nd and 6th carbons in phenol are equivalent. This means that compounds A and C are the identical ‒ they’re both 2-nitrophenol. So, we actually end up with twice as much 2-nitrophenol as 4-nitrophenol.
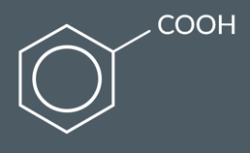
Benzoic acid (shown below) can undergo electrophilic substitution with bromine. A halogen carrier catalyst is required and the temperature of the mixture must be 100°C100 °C to speed up the reaction.
Which of the following statements about benzoic acid are true?
The pi system is more electron-rich than in benzene.
COOH is an electron withdrawing group.
Benzoic acid’s pi system attracts electrophiles more strongly than benzene’s.
Br substitutes at the 3rd carbon.
Br substitutes at the 2nd or 4th carbon.
Br substitutes at the 2nd, 4th and 6th carbon
COOH is an electron withdrawing group.
Br substitutes at the 3rd carbon
When phenol reacts with bromine, it undergoes 3 substitution reactions to form 2,4,6-tribromophenol.
Meanwhile, when phenol reacts with nitric acid, it only substitutes once, forming 2-nitrophenol and 4-nitrophenol.
Given what you know about the behaviour of the nitro group, suggest an explanation for this difference.
The nitro group is electron-withdrawing. It reduces the electron density of the pi system and decreases the attraction between the benzene ring and electrophiles.
So, once phenol has been nitrated once, the nitrophenol product is less reactive towards nitric acid and further substitution is unlikely.
This is not the case for bromine. Once phenol has reacted once, the bromophenol product is still reactive towards bromine and further substitution is likely.