Halogenoalkanes
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
What are Halogenoalkanes?
Halogenoalkanes are organic compounds where on or more halogen atoms replace the hydrogen atoms in a alkane. The general formula is CnH2n+1X, where X is the halogen. Their functional group is the C-X bond.
The C-X bond in halogenoalkanes is polar as the halogens are more electronegative than carbon (C is more positive, X is more negative). As you go down the halogen group, the bonds get less polar so the bond strength decreases.
What are Primary, Secondary and Tertiary Halogenoalkanes?
Primary - The halogen the carbon is attached to only has one carbon bonded to it.
Secondary - The halogen the carbon is attached to has two carbons attached to it.
Tertiary - The halogen the carbon is attached to has three carbons attached to it.
What are the properties of Halogenoalkanes?
Solubility - The polar C-X bonds are not polar enough to make the halogenoalkanes soluble in water. The main forces are dipole-dipole and van der Waals forces.
Boiling Point - The boiling point increases with chain length and down the halogen group. These are due to there being a larger molecule so increased van der Waals forces. Similarly to other groups, branching lowers the melting point.
What is the reactivity of Halogenoalkanes like?
When they react, the C-X bond usually breaks, with how long it takes being dependent on the C-X bond polarity and enthalpy.
Polarity - The polarity of the C-X bond would suggest that C-F is the most reactive as it is the most polar and C-I is the least polar.
Enthalpy - The bonds get weaker as they go down the group meaning C-F is the strongest bond and the least reactive, and C-I is the weakest bond and the most reactive.
Experiments confirm that C-I is the most reactive meaning that enthalpy is more important of a factor than polarity.
What is a Nucleophile and some common examples?
A nucleophile is a negative ion or a negatively charged molecule (due to a negative atom) that is able to donate a pair of electron and take part in an organic reaction by attacking an electron-deficient area in another reactant.
A nucleophile can do this as it has a lone pair of electrons situated on an electronegative atom which it can donate to form a new bond.
Common Examples:
-OH (Lone pair on the O)
NH3 (Lone pair on the N)
-CN (Lone pair on the C)
They replace the halogen in a halogenoalkane in a reaction called nucleophilic substitution.
What is Nucleophilic Substitution?
A reaction where a nucleophile attacks a compound and replaces the leaving group, usually a halogen.
Leaving Group - An atom or group of atoms that is ejected from the starting compound, often taking an electron pair to form a negative ion.
A substitution between bromoethane and a cyanide ion is shown in the image.
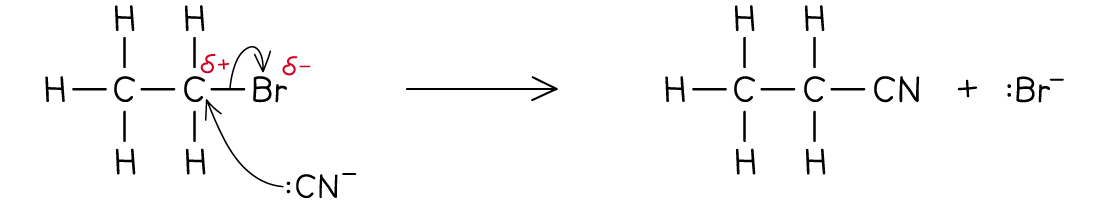
What are some examples of Nucleophilic Substitution.
Halogenoalkanes with cyanide ions - Forms a nitrile and a halogen ion, the main compound also now has an extra carbon atom.
Halogenoalkanes with hydroxide ions - Forms a alcohol and a halogen ion
Halogenoalkanes with Ammonia - Forms an amine, a halogen ion and a Ammonia ions (NH4+)
Ammonia does not have a negative charge so it is a neutral nucleophile. This means that a positive ion will be left on the main compound so a proton must be lost by reacting with a second ammonia molecule forming NH4+ (See Image:)

What is a Base?
A base is a substance that can accept hydrogen ions (protons) and are useful in elimination reactions.
What are Elimination Reactions in Halogenoalkanes?
A reaction where a molecule loses a hydrogen halide, leaving a double bond in its place so it can form an alkene. These reactions usually occur with a base.
Under the right conditions, -OH can act as a base, eliminating a hydrogen halide.
The conditions needed for a elimination reaction are a hydroxide (usually Sodium or Potassium) is dissolved in ethanol and mixed with the halogenoalkane. The mixture is then heated, producing ethene, which can be tested by collecting the gas and burning it and seeing if it decolourises bromine solution.
What is the mechanism of a Elimination Reaction?
If the base is a hydroxide ion, then a alkene, water molecule and halogen ion is formed (See Image)
If the base is a sodium/potassium hydroxide molecule, then a alkene, water molecule and potassium halide molecule is formed.

When is a reaction a Substitution or Elimination?
A hydroxide ion will react with halogenoalkanes as a nucleophile or as a base depending on the conditions. this depends on two factors: The type of Halogenoalkane (Primary, Secondary, Tertiary) and the Reaction Conditions (Aqueous or Ethanolic Solution)
Reaction Conditions:
Hydroxide Ions at room temperature, dissolved in water (aq), favour substitution.
Hydroxide ions at high temperature, dissolved in ethanol, favour elimination.
Type of Halogenoalkane:
Primary/Secondary Halogenoalkanes react by substitution.
Secondary/Tertiary Halogenoalkanes react by elimination.
What are Chlorofluorocarbons (CFCs)?
They are halogenoalkanes containing both chlorine and fluorine atoms but not hydrogen (CCl3F). They are very unreactive under normal conditions .
Short chain CFCs are gases and were used as refrigerants and aerosol propellants. Longer chain CFCs are used as dry cleaning solvents.
How do CFCs affect the Atmosphere and the Ozone Layer?
Ozone is a molecule O3 that decomposes to oxygen, and at ground level can cause smog and respiratory issues.
The Ozone Layer is important as it protects the Earth from exposure to too many UV rays by absorbing a significant amount of ultraviolet radiation from the sun.
CFCs form chlorine free radicals when the C-Cl bond breaks down homolytically in the presence of UV radiation which then attack ozone molecules: Cl• +O3 → ClO• + O2
The resulting free radicals also attack ozone and regenerate Cl•: ClO• + O3 → 2O2 + Cl•
By adding these two equations together (2O3 → 3O2) you can see that chlorine radicals act as a catalyst here and are not destroyed.
CFCs are now being phased out with new CFC free solvents to prevent them entering the atmosphere.