Immunology Critical Competencies
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
*Identify 2 examples of each of the two branches of immunity, natural (innate) and acquired (adaptive) immunity. Describe why each example fits in each category
Innate:
External defenses: prevents infectious agents from entering the body
unbroken skin
mucosal membrane surfaces
secretions (lactic acid, fatty acid, psoriasin, lysozymes)
cilia in the nasopharyngeal passages
urine (system, pH, lactic acid)
coughing/sneezing
microbiota
Internal defenses: Destroys foreign invaders that have penetrated the barriers of the external defense system
Phagocytic cells
soluble factors
Adaptive:
Cytotoxic T cells
T helper cells
B cells
what are the two branches of immunity?
innate immunity
“natural immunity”
Adaptive immunity
Describe innate immunity
the ability to resist infection by means of normally present body functions
A nonspecific, immediate response that lacks memory and specificity
Cells involved…
leukocytes (neutrophils, basophils, eosinophils, monocytes)
Tissue cells (macrophages, mast cells, dendritic cells)
Describe adaptive immunity
resistance characterized by specificity for each individual pathogen, or microbial agent, and the ability to remember a prior exposure
Cells involved…
lymphocytes (T cells, B cells, and innate lymphoid cells)
Describe characteristics of active and passive immunity.
Active immunity:
Immunity resulting from natural exposure to an infectious agent or administration of a vaccine
Results in long-term immunity (memory cells and antibodies)
Examples…
Exposure to a pathogen
vaccines
Passive immunity:
Immunity that results from the transfer of antibodies from immunized hosts to a nonimmune individual
results in short-term, immediate protection to someone who has not developed immunity
Examples…
antibodies from mother’s breast milk to child
Commercial antibody administration (immunotherapy)
Discuss foreignness and its role in the antigenicity of a molecule.
Foreignness = un-relatedness to the host
Lymphocytes do not respond to self-antigens (only foreign antigens)
The more foreign (taxonomic difference), the more antigenic. The similar it is to the host antigens, the less antigenic.
Examples:
Plant protein is a greater immunogen for an animal than material from a related animal
Discuss molecular size and its role in the antigenicity of a molecule.
Generally, the greater the molecular weight, the more potent the molecule is as an immunogen
Immunogens are generally at least 10,000 Daltons in order to be recognized by the immune system
Most effective immunogens are at least 100,000 Daltons
Discuss chemical structure complexity and its role in the antigenicity of a molecule.
The greater the chemical composition and molecular complexity, the more immunogenic it is.
Proteins and polysaccharides are more immunogenic
Synthetic polymers like nylon or teflon are less immunogenic
made up of a few simple repeating units (no bending or folding)
Proteins are highly immunogenic compared to polysaccharides, nucleic acids, and lipids due to their interaction with T and B cells
Discuss epitope and its role in the antigenicity of a molecule.
The small portion of an immunogen that is recognized by a lymphocyte.
Epitopes come in precise shapes that trigger the production of a specific antibody or activate a T-cell.
Some larger molecules can have multiple epitopes, which enables more responses.
The number and accessibility of epitopes determine the strength of the immune response.
Discuss dosage and its role in the antigenicity of a molecule.
Refers to the amount of antigen introduced into the body.
If the antigen is introduced in a small quantity, it could potentially not be detected by the immune system.
Too much antigen can risk overwhelming the immune system.
The optimal dosage is essential for supplying enough antigen required to elicit an immune response without overwhelming the body.
Discuss route and timing of exposure and its role in the antigenicity of a molecule.
Routes of exposure include intravenous, intradermal, subcutaneous, intramuscular, and oral.
Each route shuttles antigens to different secondary lymphoid tissues, where they encounter different immune cells.
The interaction of the antigen with immune cells affects how strong the response is.
Primary or secondary exposure influence timing and strength of response.
Describe the interaction between antigens and antibodies including how the term lock and key fit
Epitopes are recognized by antibodies and are described as fitting a key in a lock
The antigen is the key, and the antibody is the lock
This interaction described how antibodies distinguish between many different antigens with high accuracy
Describe the basic structure of immunoglobulins. Include the following terms:
a. Light and heavy chains
b. Kappa and lambda chains
c. Alpha, mu, delta, epsilon, gamma
d. Constant, variable and hyper-variable regions
e. Hinge region
f. J chain
g. Disulfide bonds
h. Fab and Fc
i. Pentamer, dimer, and monomer
Each immunoglobulin contains peptide chains of varying sizes bound together, known as the heavy and light chains. Two large peptide chains (heavy) are bound to two smaller peptide chains (light) to make up the body of the molecule. Each immunoglobulin class is characterized by its heavy chain. Each of the five heavy chains has a different molecular weight and is denoted by the Greek letters alpha, mu, delta, epsilon, and gamma. For instance, IgG is made up of two Gamma heavy chains, IgA is made up of two alpha heavy chains, IgM is made up of two mu heavy chains, IgE is made up of two epsilon heavy chains, and IgD is made up of two delta heavy chains. However, each immunoglobulin shares the same structure for its light chains. Two light chains can either be kappa or lambda chains. There are only minor differences between the two light chains, but they have no functional differences. Heavy and light chains are held together by disulfide bonds, and their exact number differs between immunoglobulin classes. Each chain contains a single variable region and one or more constant regions. The variable region, located at the amino terminal, is unique to each antibody molecule and allows for specific antigen binding. The constant region, located towards the carboxyl terminal, is the same for each immunoglobulin and is responsible for immune defense. The molecule is divided into a Fab region and an Fc region, held together by the hinge region. The Fab fragment is the area capable of binding antigens, while the Fc region is responsible for interacting with other cells. The hinge region allows the immunoglobulin to be flexible during cellular interactions. Located at the tips of the Fab regions are the hyper-variable regions, which form the antigen-binding site. This generic Y shape is referred to as a monomer, because it is a single Y-shaped unit. However, some antibodies are capable of joining together to form larger structures. A dimer is two monomer units joined together by the J chain, while a pentamer is five monomer units joined together by a J chain.
Define isotype
A unique amino acid sequence that is common to all immunoglobulin molecules of a given class in a given species
(heavy chain that is unique to each Ig class)
(what makes each Ig different)
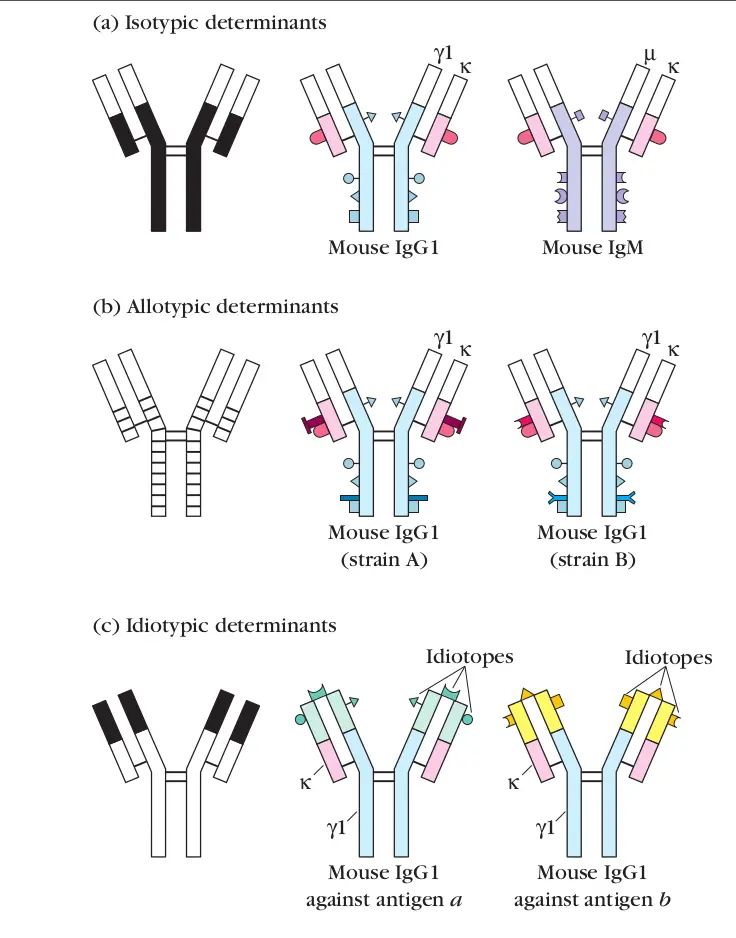
Define allotype
a minor variation in amino acid sequence in a particular class of immunoglobulin molecules that is inherited in Mendelian fashion
(genetic variations in the constant regions)
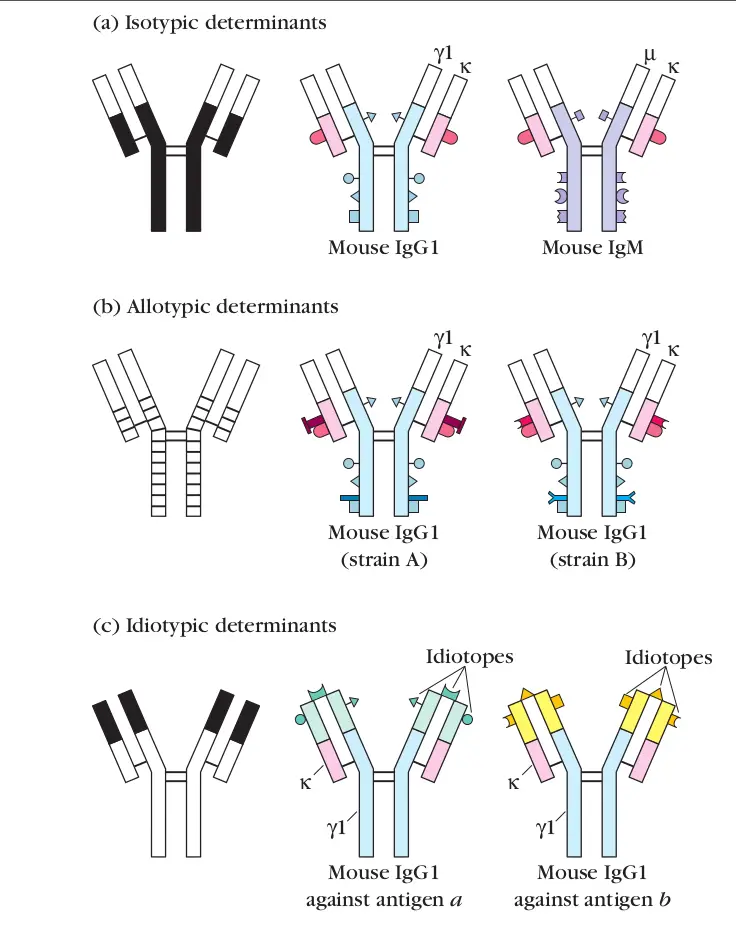
Define idiotype
the variable portion of light and heavy immunoglobulin chains that is unique to a particular immunoglobulin molecule. This region constitutes the antigen-binding site
(Variations in variable regions that give the molecule specificity)
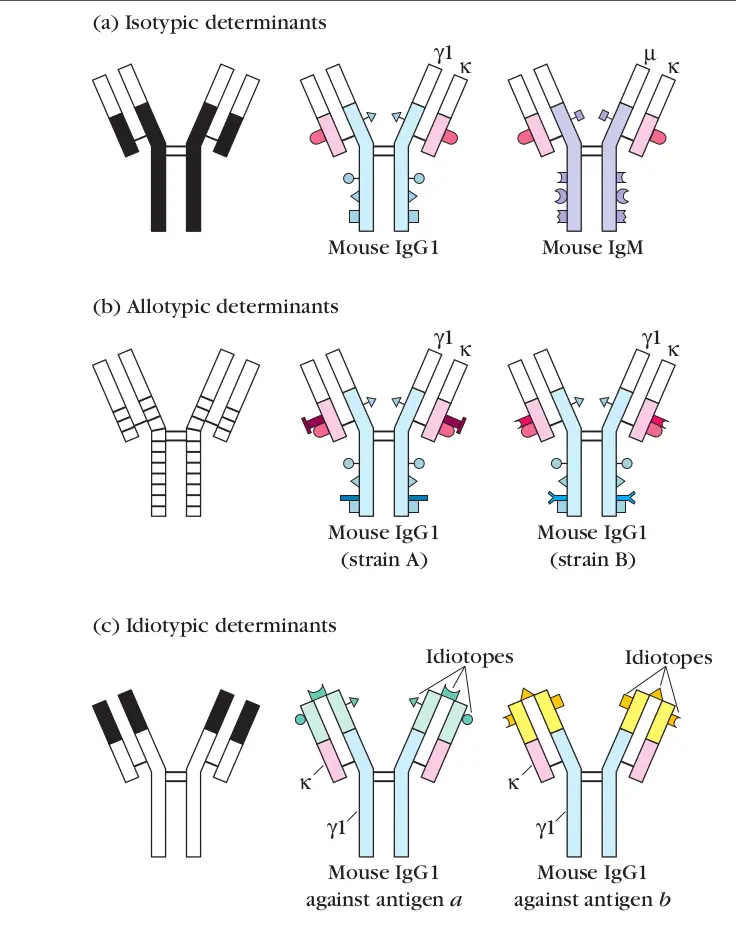
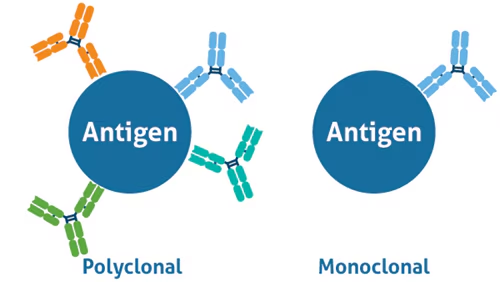
Define monoclonal antibody
very specific antibody derived from a single antibody-producing cell that has been cloned or duplicated
(Identical antibodies produced by a single B-cell clone)
(bind to one specific epitope on an antigen)
(made in labs)
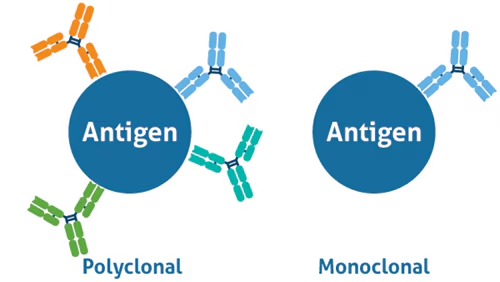
Define polyclonal antibody
Derived from many clones of cells (B cells or plasma cells), and are therefore diverse in terms of their antigen specificity
(A mixture of antibodies produced by different B-cell clones
(bind to multiple epitopes on the same antigen)
(made naturally by the body)
Serum concentration of the five immunoglobulins
IgG = 800-1600 mg/dL or 70-75% total serum
IgA = 70 - 350 mg/dL or 10-15% total serum
IgM = 120 - 150 mg/dL or 5-10% total serum
IgE = 0.005 mg/dL or 0.0005% total serum
IgD = 1-3 mg/dL or 0.001% total serum
List the relative size for the five immunoglobulins (MW)
IgG = 1500,000
IgA = 1600,000
IgM = 900,000
IgE = 190,000
IgD = 180,000
List the valency for the five immunoglobulins
IgG = 2
IgA = 2 (monomer) and 4 (dimer)
IgM = 10
IgE = 2
IgD = 2
List the location in the body for the five immunoglobulins
IgG = blood, placenta, tissue fluids, lymph
IgA = Mucosal surfaces, serum, breast milk, saliva, tears, and sweat. Found primarily in MALT
IgM = blood vessels
IgE = Lungs, skin, lining of the respiratory and alimentary tracts
IgD = found on the surface of immunocompetent unstimulated B lymphocytes
List the temperature of activity for the five immunoglobulins
IgG = 37 degrees C
IgA = 37 degrees C
IgM = below 25 degrees C
IgE = 37 degrees C
IgD = 37 degrees C
List the ability to cross the placenta for the five immunoglobulins
IgG = Yes
IgA = No
IgM = No
IgE = No
IgD = No
List the complement activation for the five immunoglobulins
IgG = strong ability to bind complement
IgA = May trigger the alternate complement pathway but not capable of fixing complement by the classical pathway
IgM = most efficient at triggering the classical pathway
IgE = does not bind complement
IgD = does not bind complement
List the affinity/avidity for the five immunoglobulins
IgG = High
IgA = moderate to high
IgM = low affinity, high avidity
IgE = high affinity for FCeRI
IgD = Low