Coordination Compounds
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
What is the relationship between the shape of a molecule and coordination number?
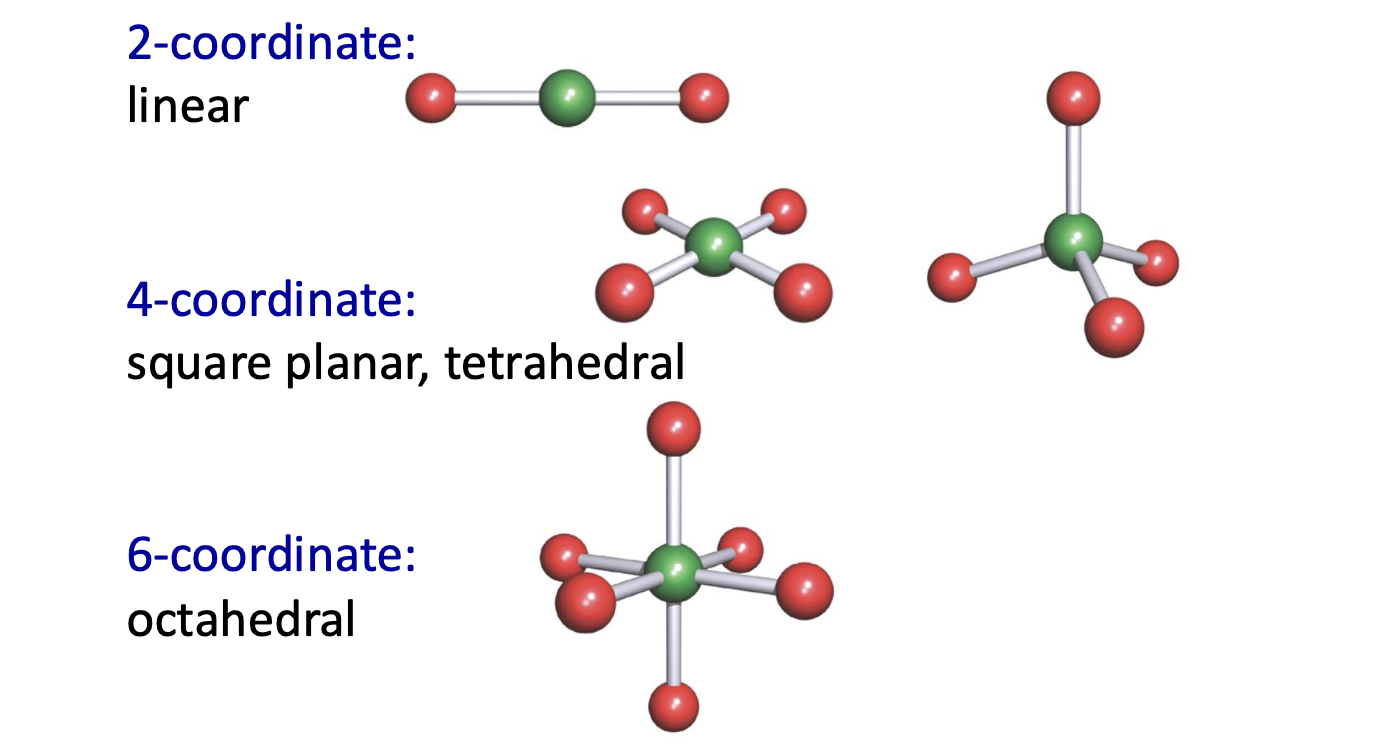
What is the relationship between charge of the ligand and the charge of the metal ion?
TO determine that NH3 was neutral, you cand raw the Lewis structure.

What is dentisiy?
Denticity refers to the number of donor atoms in a single ligand that bind to a central atom in a coordination complex. The greater the denticity, the ‘stronger’ the complex.
Monodentate, bidentate, polydentate.
Specific atoms within the ligand molecule that have a lone pair of electrons they can share (donate) with the central metal ion to form coordinate bonds. They can be the same element.
What are unidentate ligands?
Ligands that attach to the metal ion through a single donor atom.
Can only make one coordination bond.
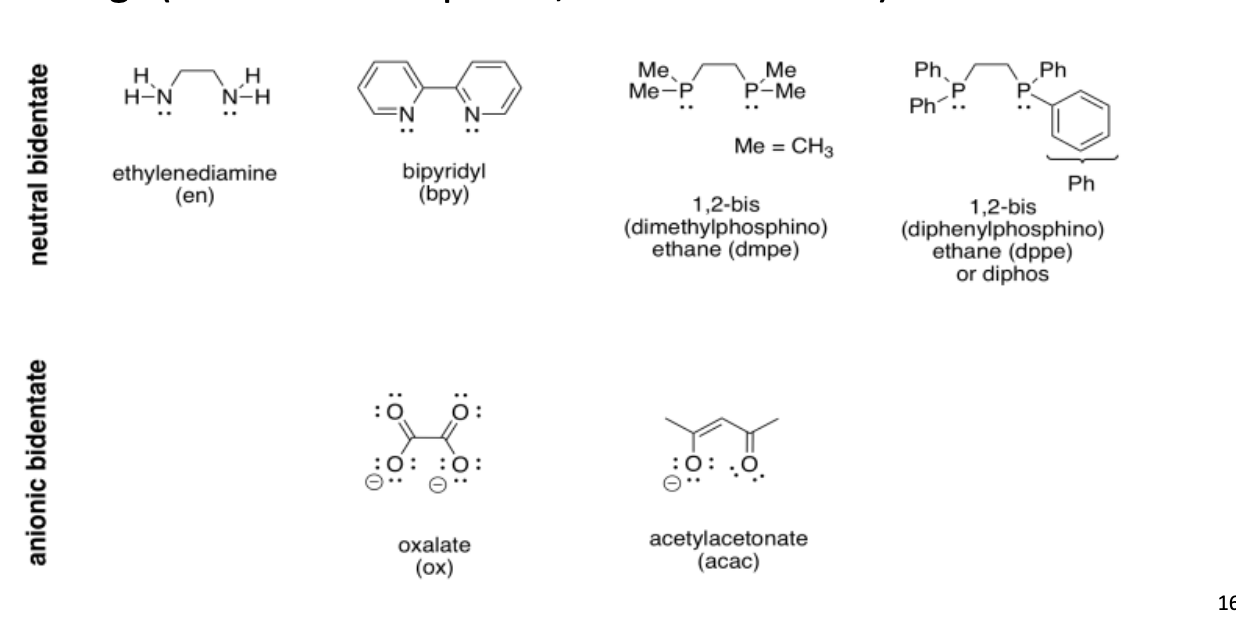
What are bidentate ligands?
They have two points of attachment to the metal atom.
Ethylenediamine has two lone pairs of electrons far away from each other which can form two coordination bonds.
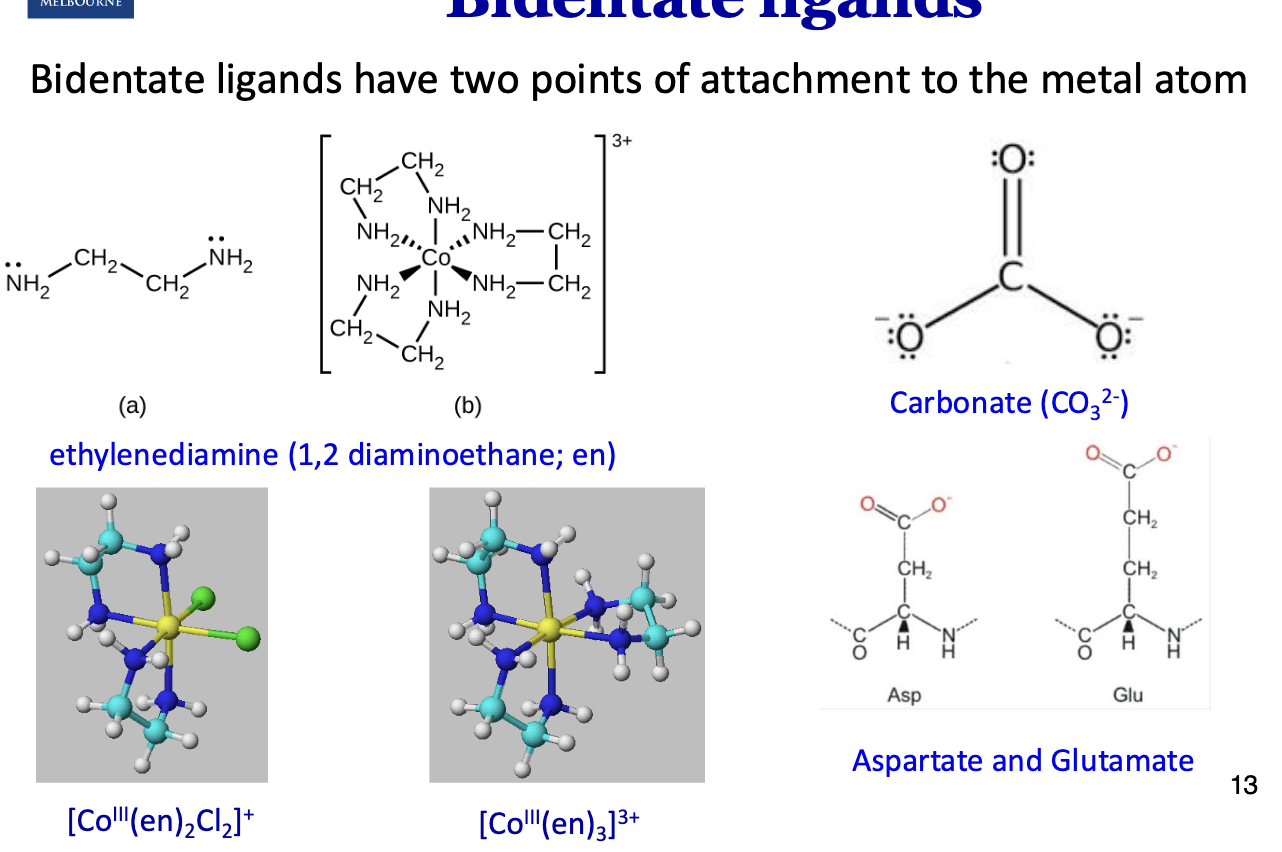
What are chelate complexes?
When a ligand with a greater dentisity >1 binds to a metal ion, they form chelate rings (chelate=claw).
What is the chelate effect?
Chelate ligands have greater affinity to a metal ion compared with its monodentate counterparts.
Top reaction is more likely to occur than bottom.
The ring which is formed is more stable.
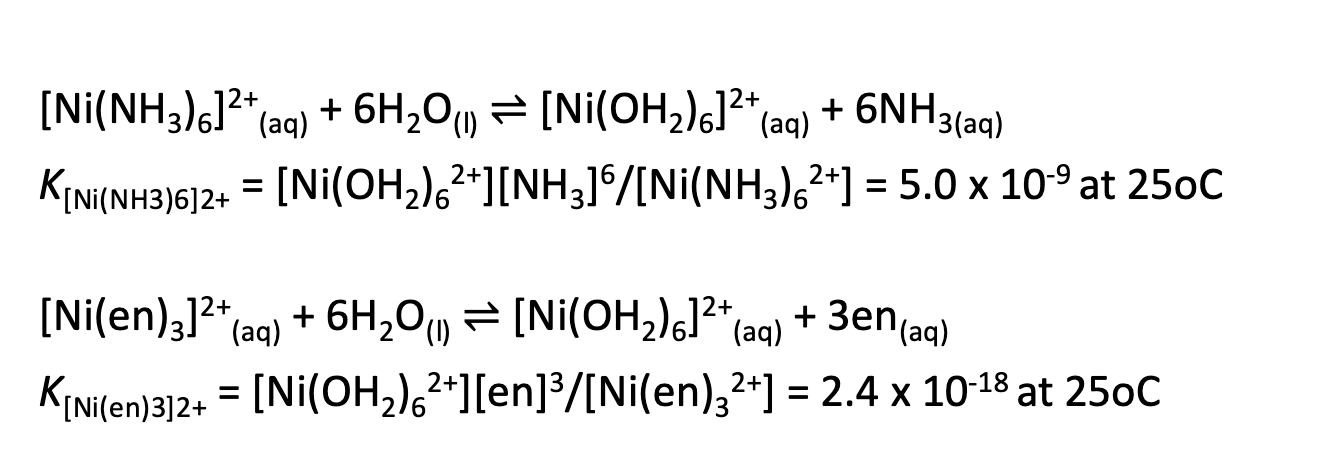
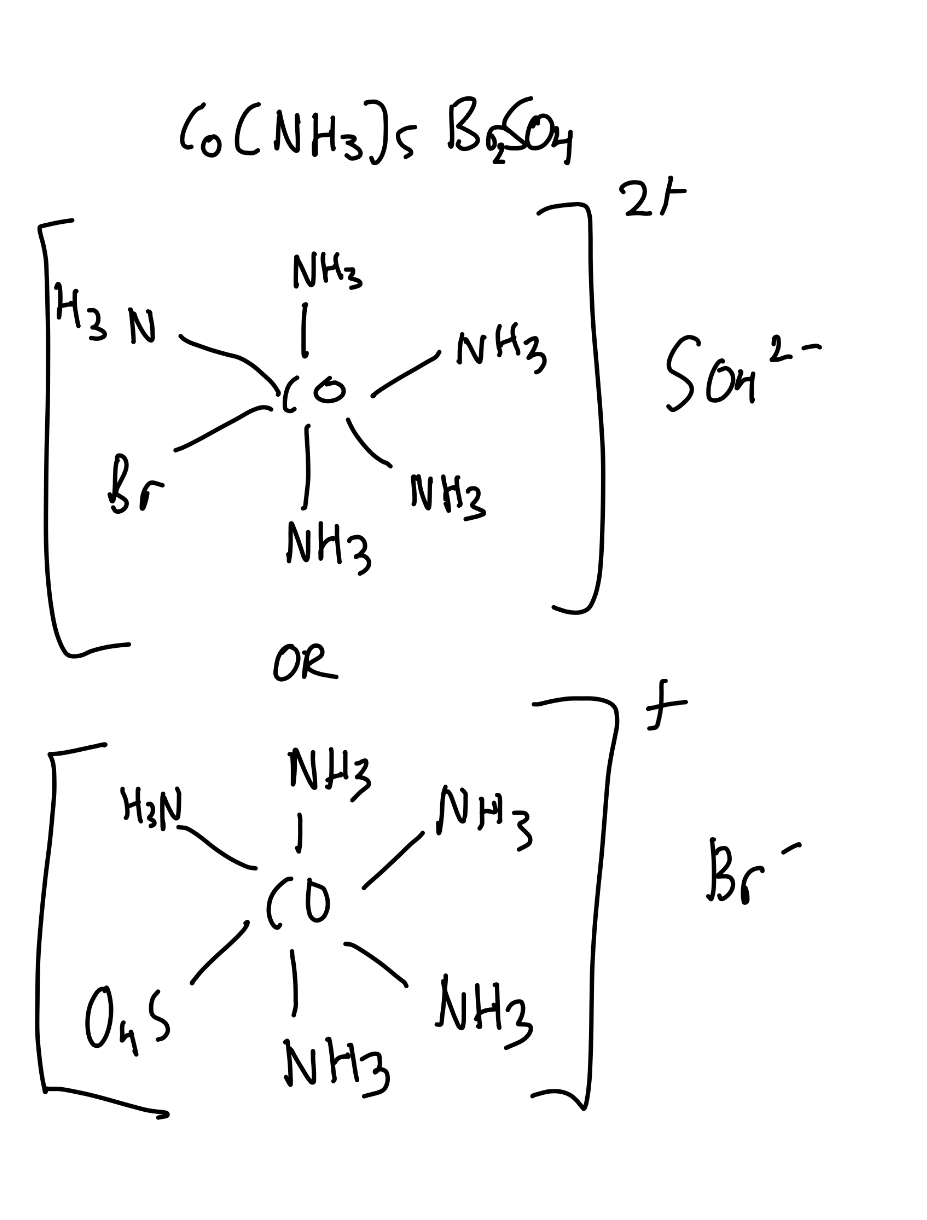
What is coordination isomerism? Do it for Co(NH3)5BrSO4
BaSO4 generates a white precipitate, whilst AgBr gives no precipitate.
If we add BaCl2, if a white precpitate forms, we can deduce that Br formed a coordination bond with CO. Coordination bonds are stronger than ionic bonds, thus the bond isn’t disturbed.
What is linkage isomerism?
THe complex has the smae formula, but ligand binds through different donate ligands.

What are sterioisomerers?
Same bonds, but different spatial arragements.
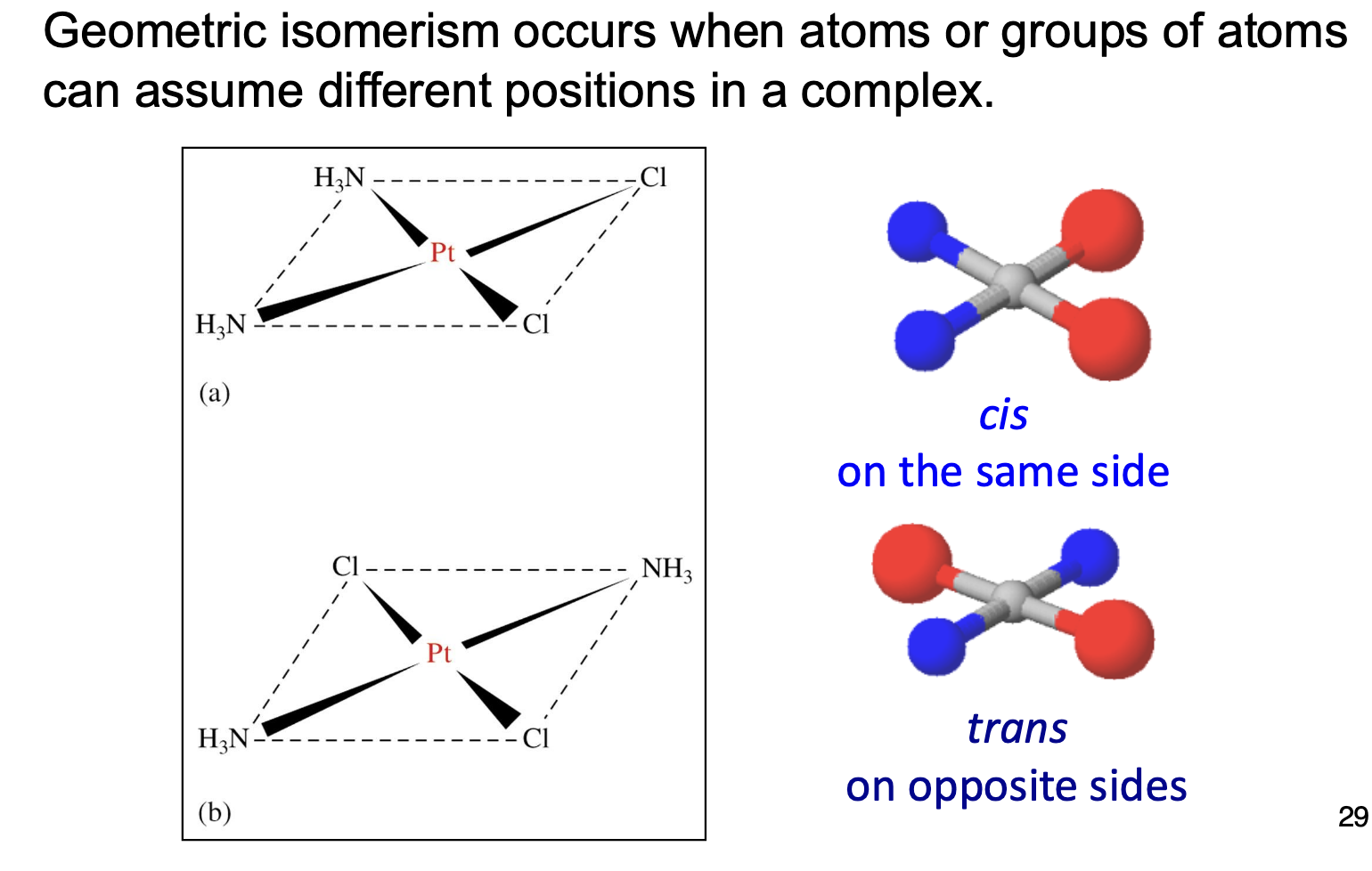
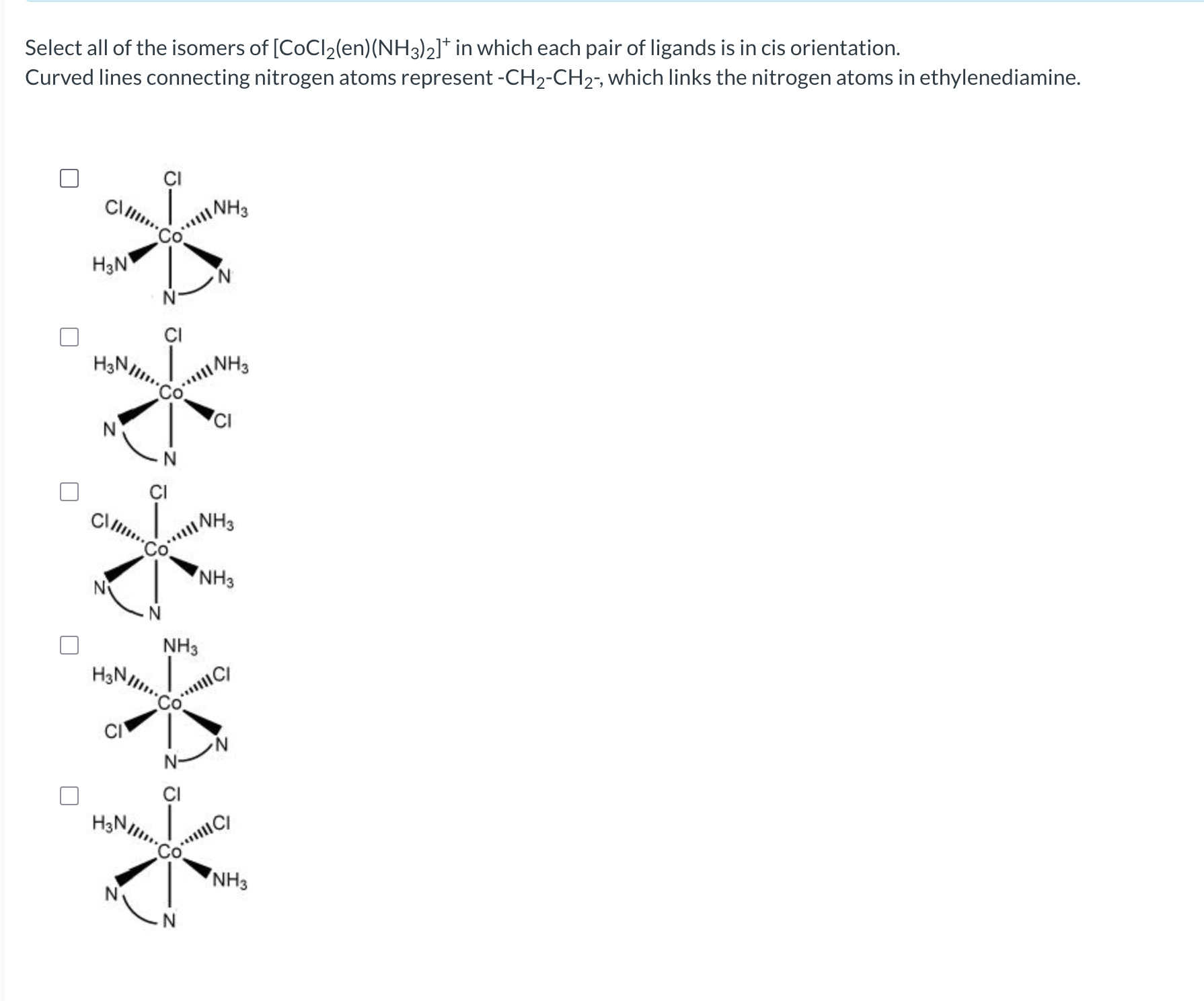
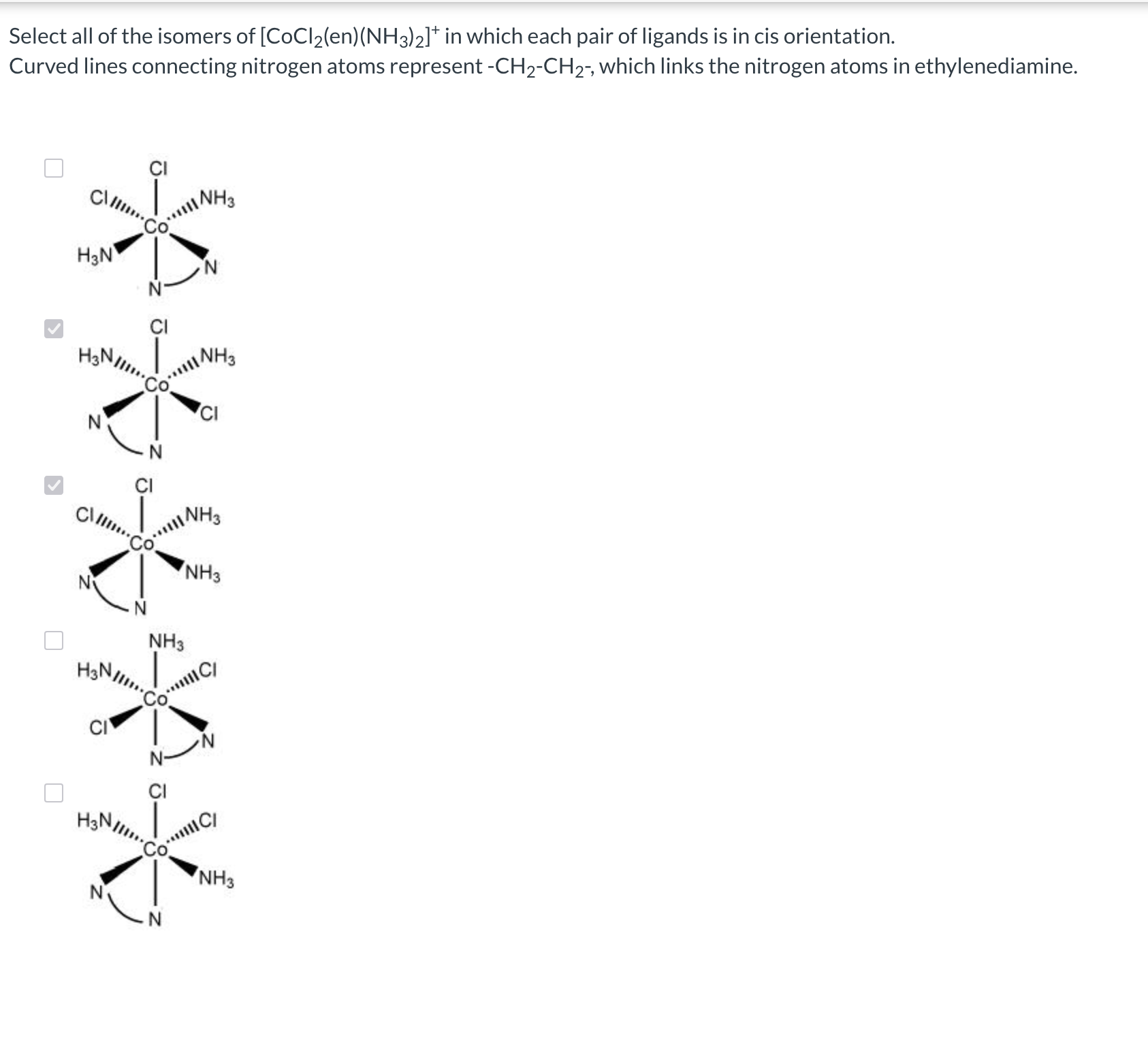
What is geometric (cis-trans) isomerism?
If they are next to each other, it is called cis, if they are opposite, it is called trans.
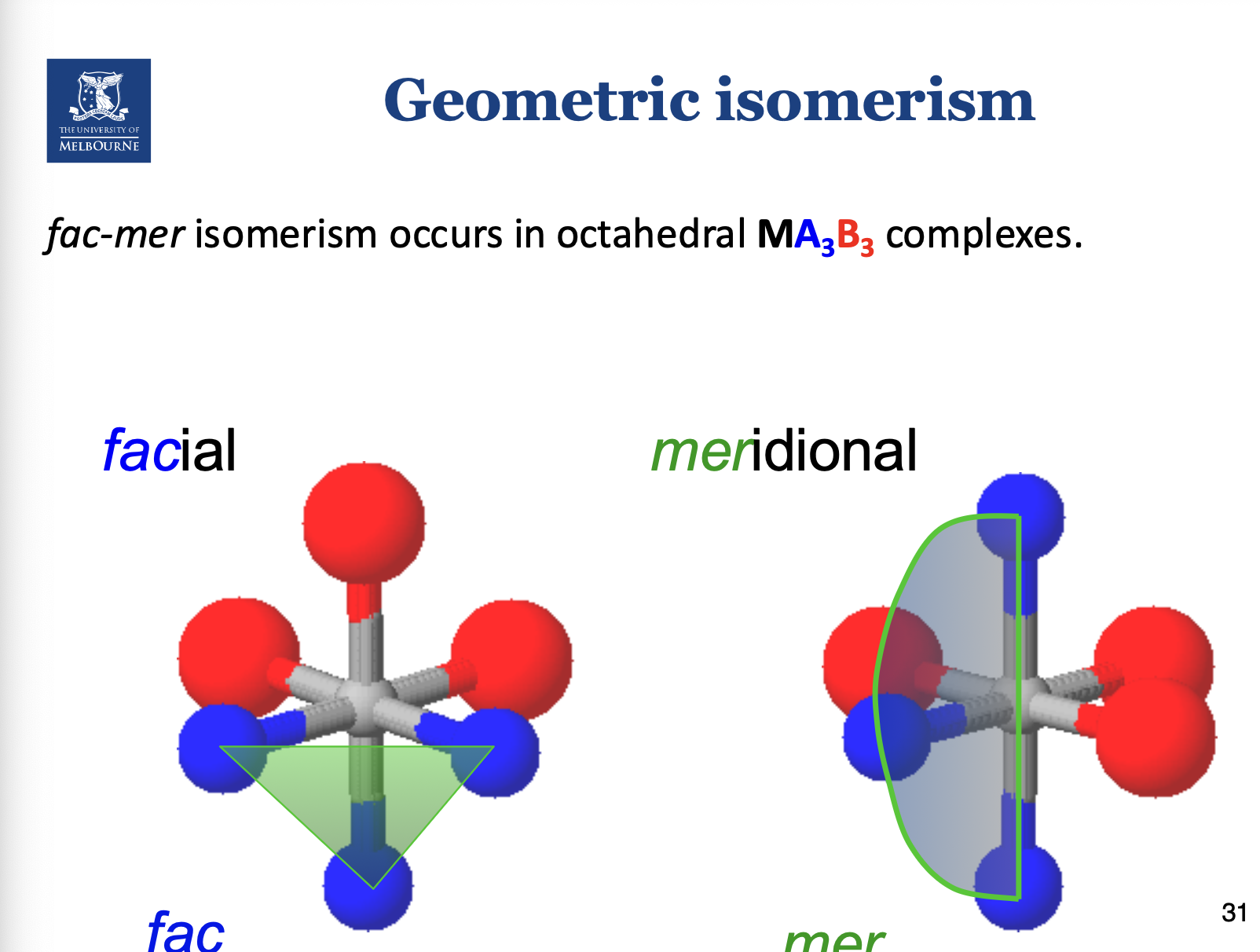
What are fac-mer isomers?
Occurs if you have 3 of the same isomers.
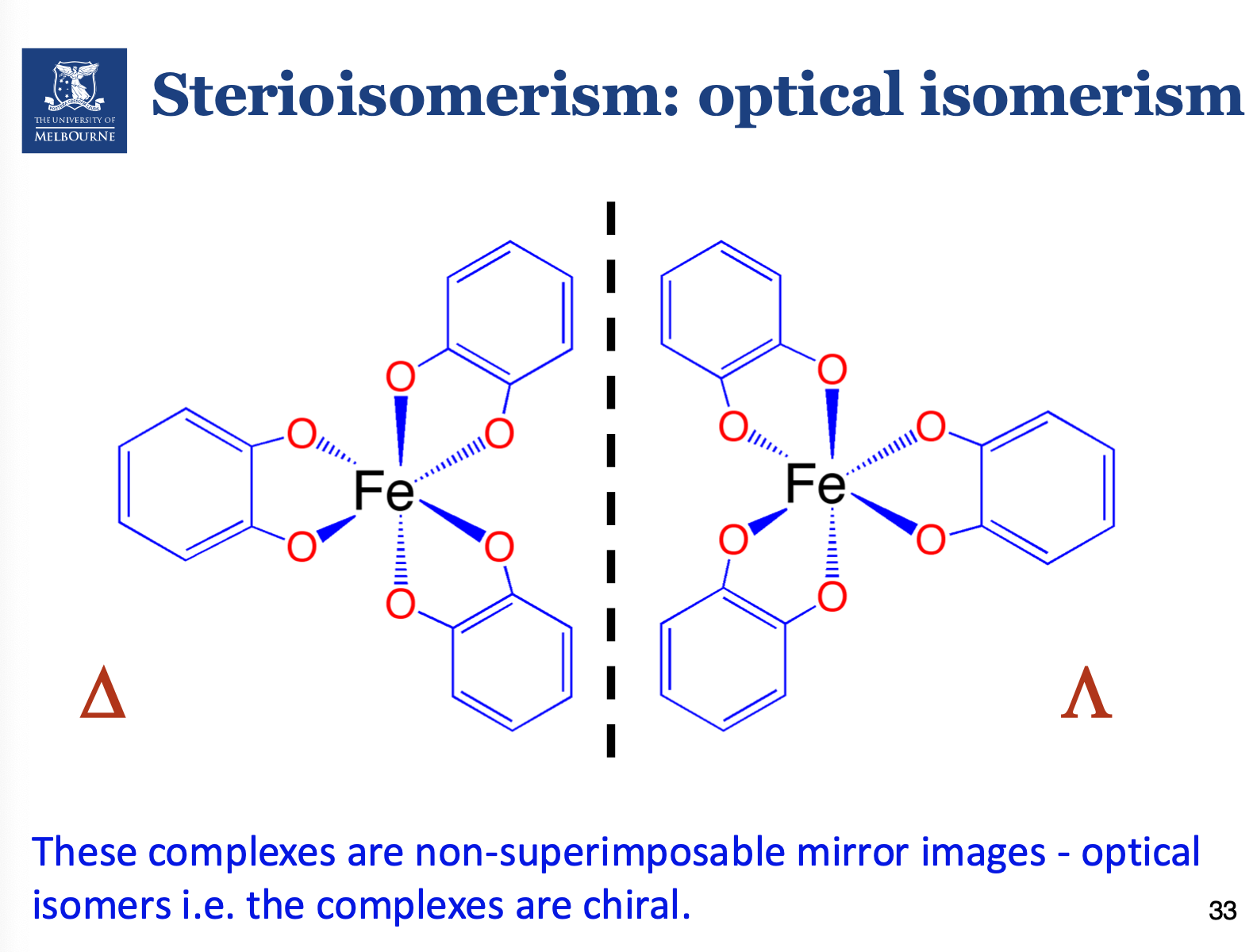
What are optical isomers?
Under acidic and basic conditions, what type of protein is favoured?
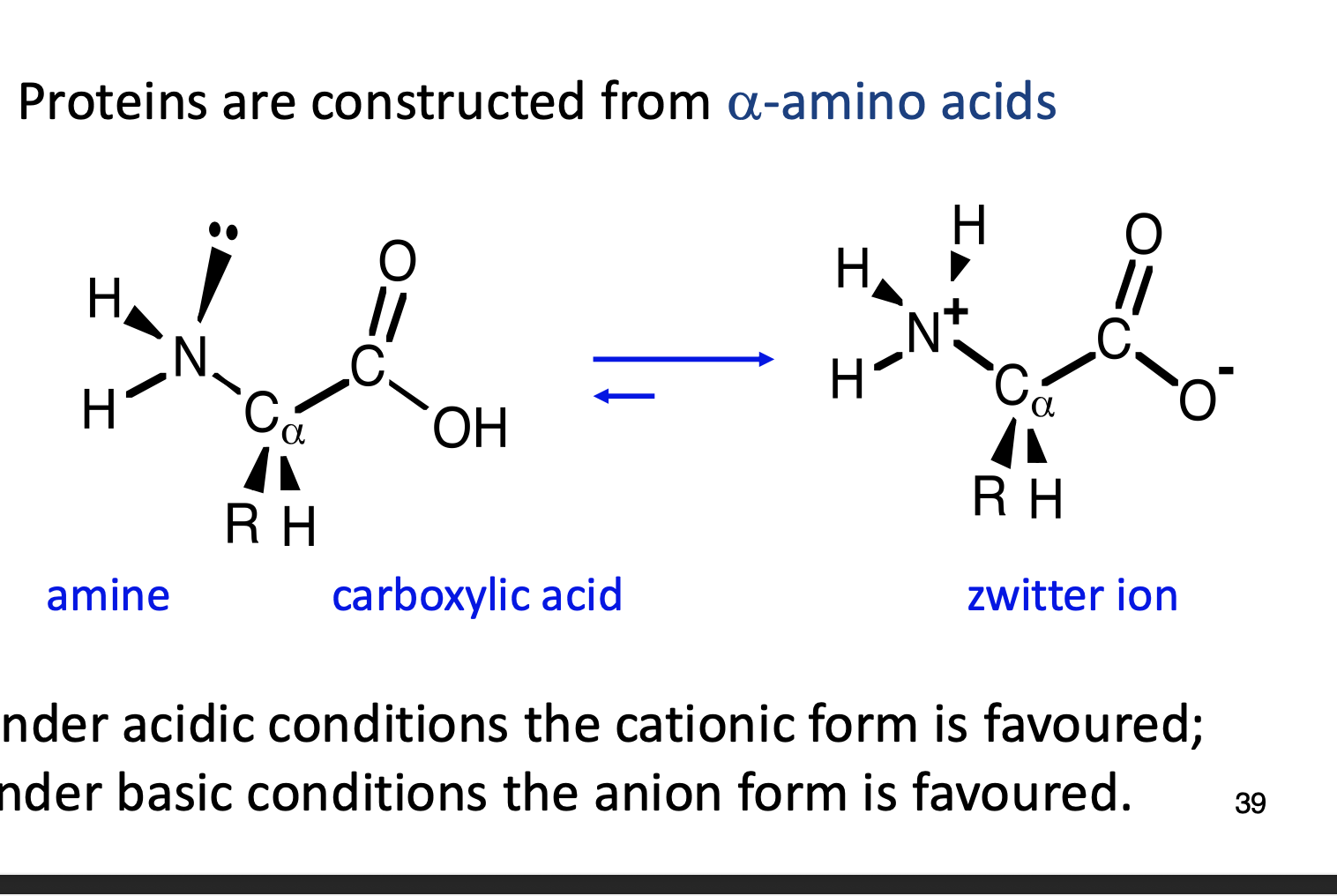
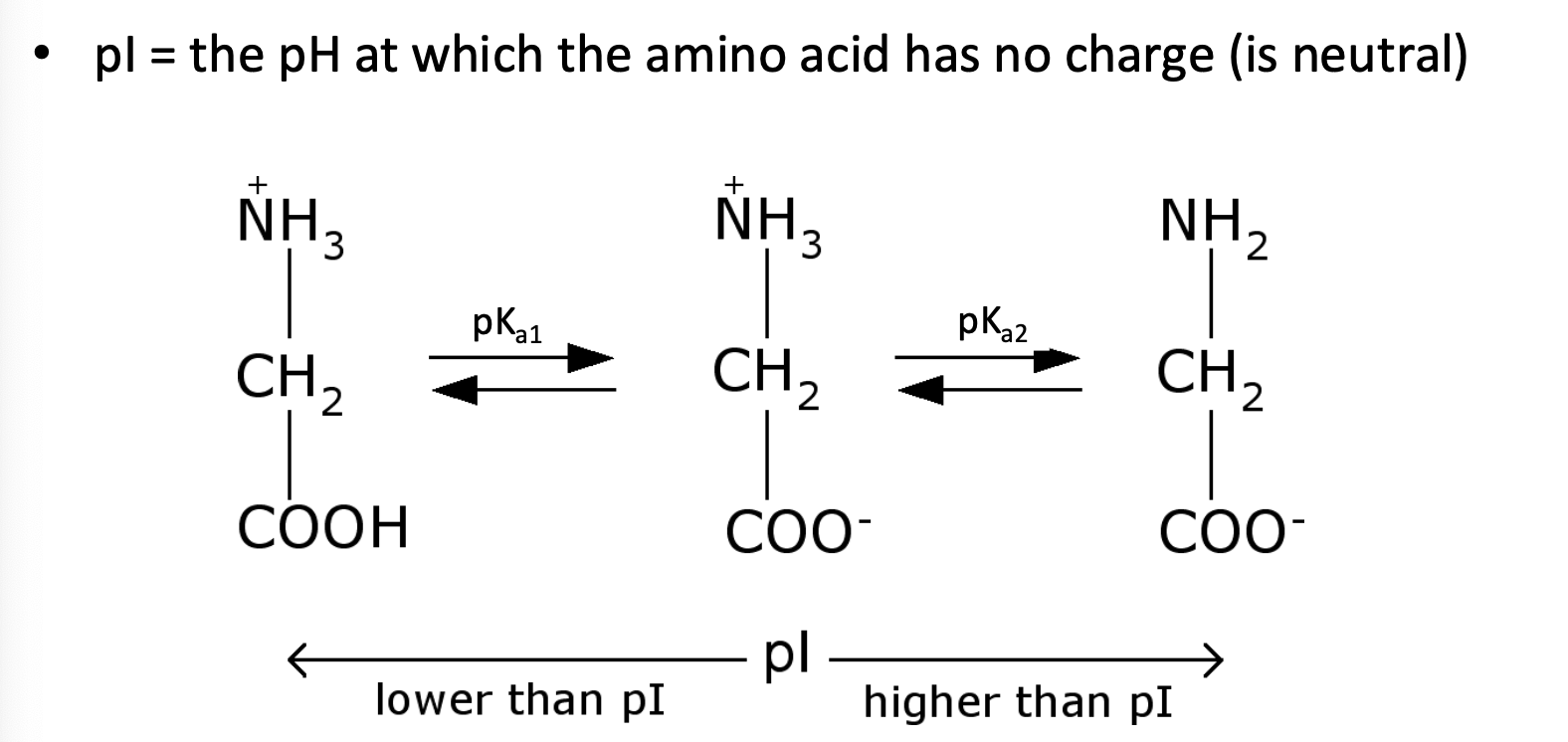
What is pl?
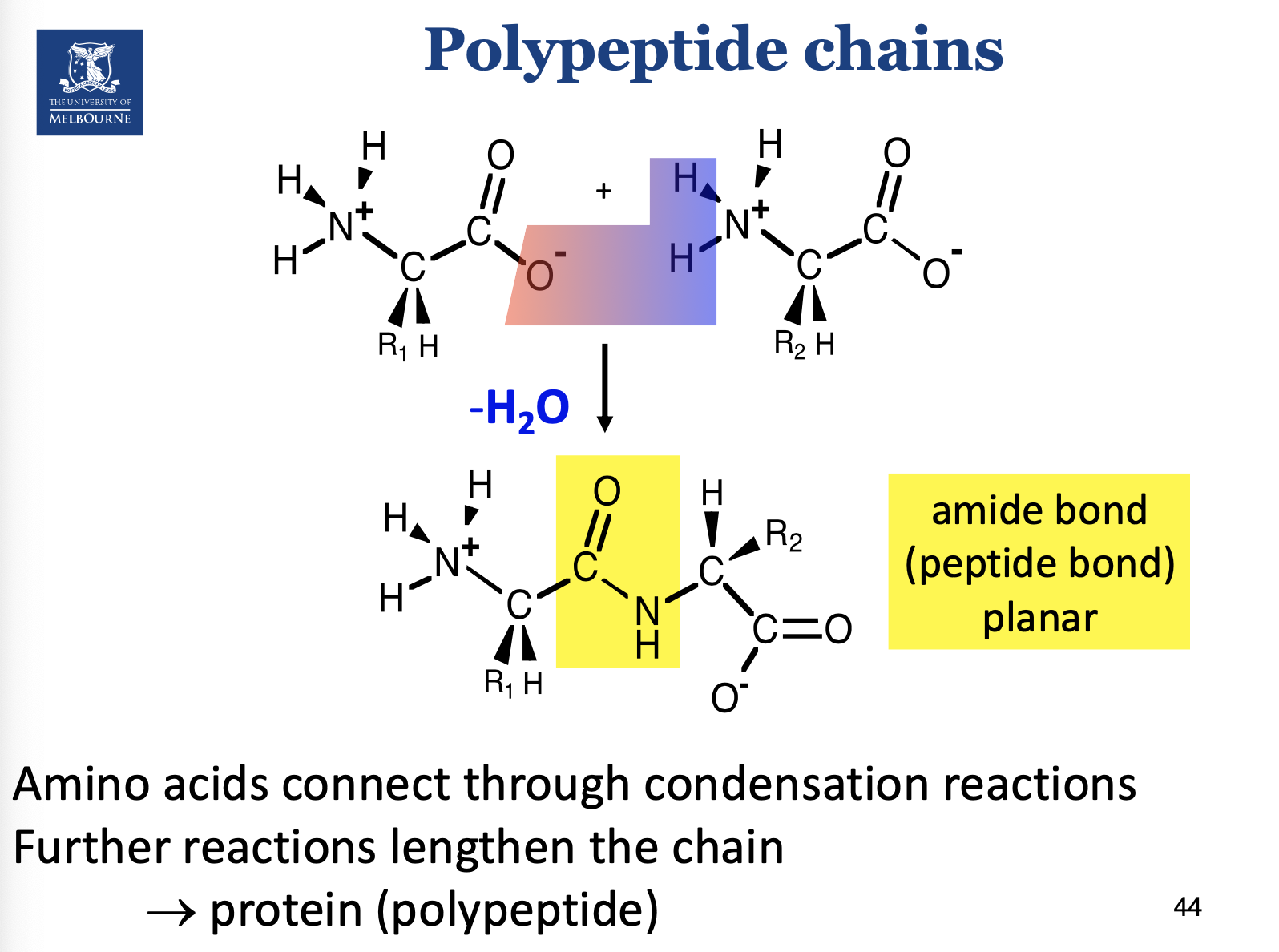
What type of bond is formed as a result of joining AA together?
Just say peptide bond.
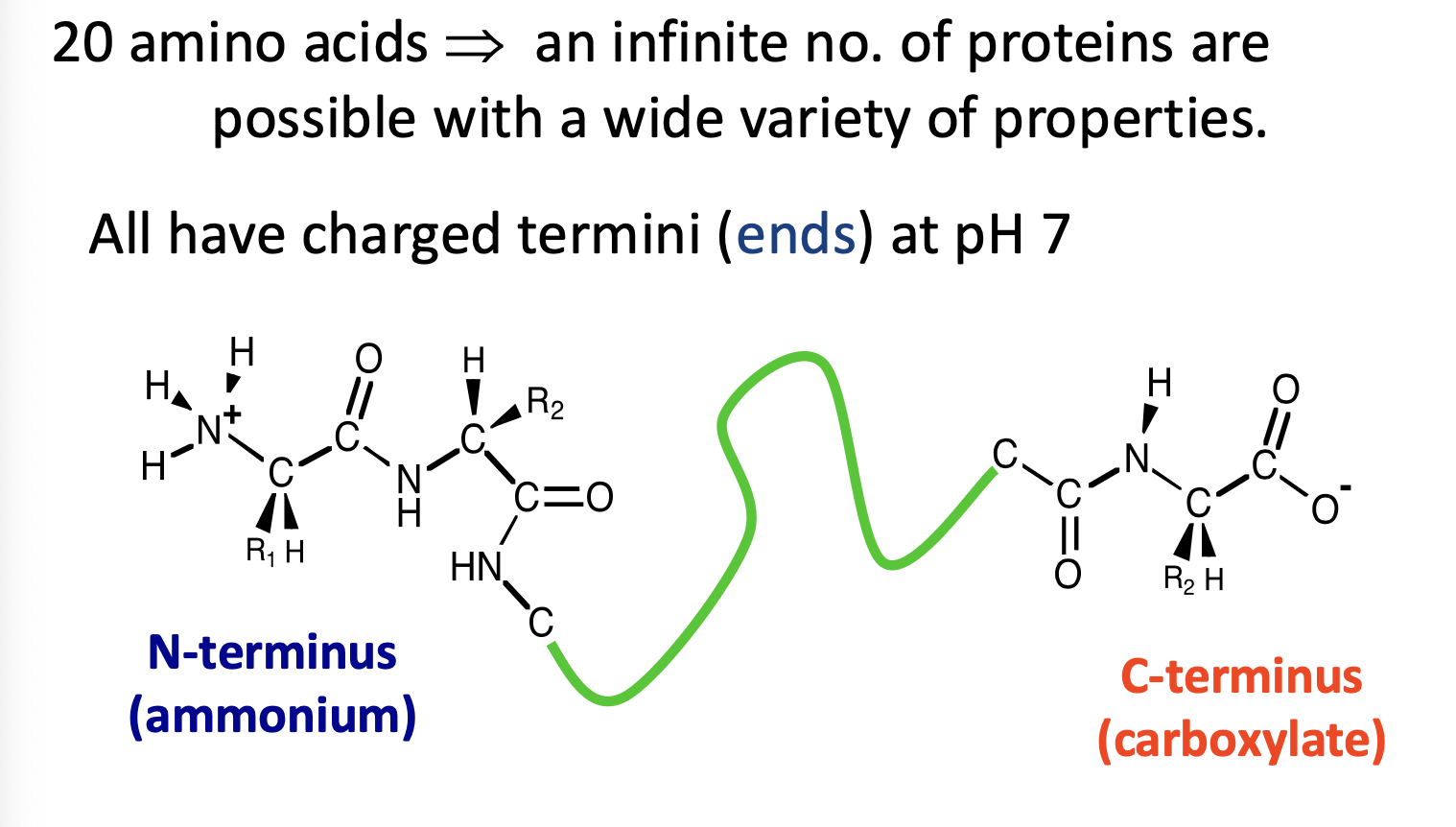
What are the terminus’ in proteins?
Always from N to C terminus.
Always charged.
What classes something as a peptide or a protein?
Peptide: 2-40AA
Protein: 40+ AA
All are PP.
What is quaternary structure?
All proteins have quaternary structure???
Can be monomeric, dimeric, etc.
What is the difference between the tertiary structure and quaternary structure of a monomeric protein?
Tertiary structure: describe the shape of the protein.
Quaternary structure: it is monomeric.
Draw Gly-Cys-Phe-His at pH 7
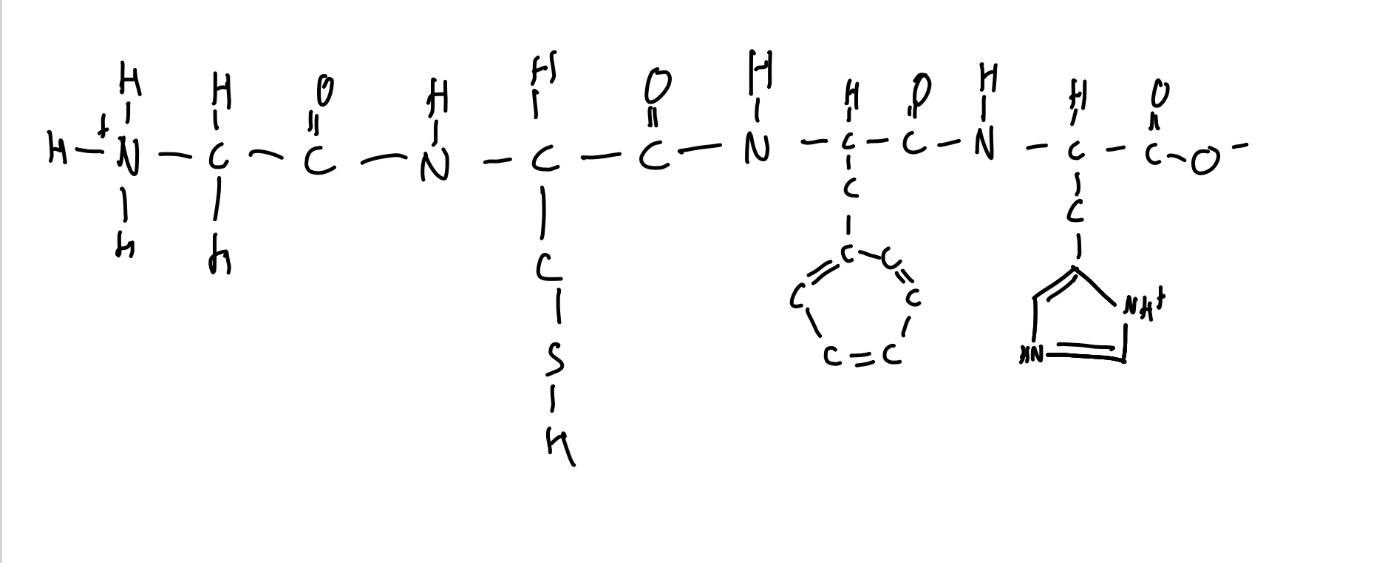
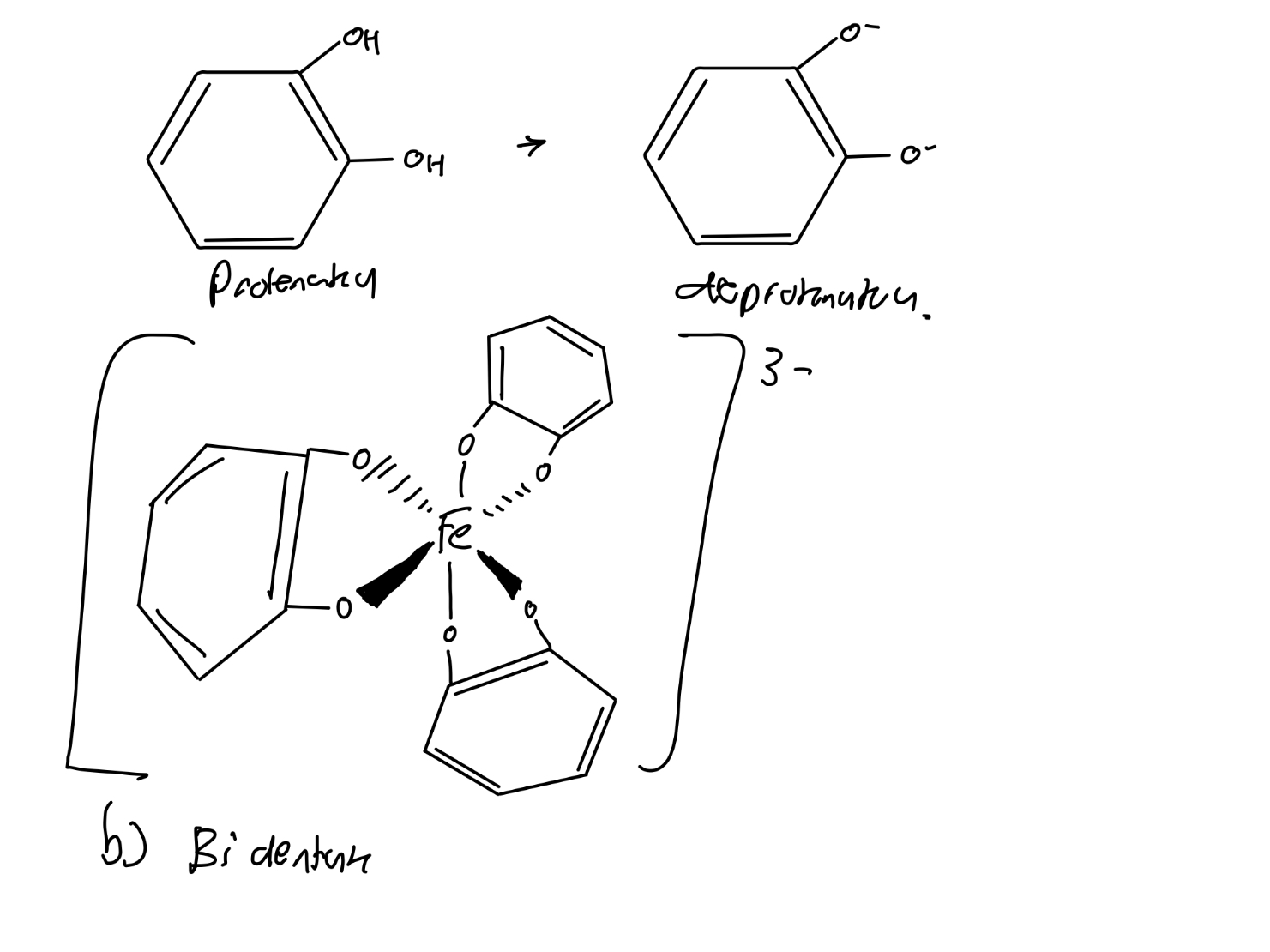
What are siderophores?
Siderophores are chelating molecules secreted by micro-
organisms that are able to bind iron ions very strongly.
• Enterobactin is a prime example of a siderophore.
• It is lipophilic which means it is able to cross cell membranes.
• It transfers Fe into cells.
How is iron obtained from the environment by bacteria?
Ligands can be used to bind and solubilize iron from the environment
For enterobactin, coordination number is 6.
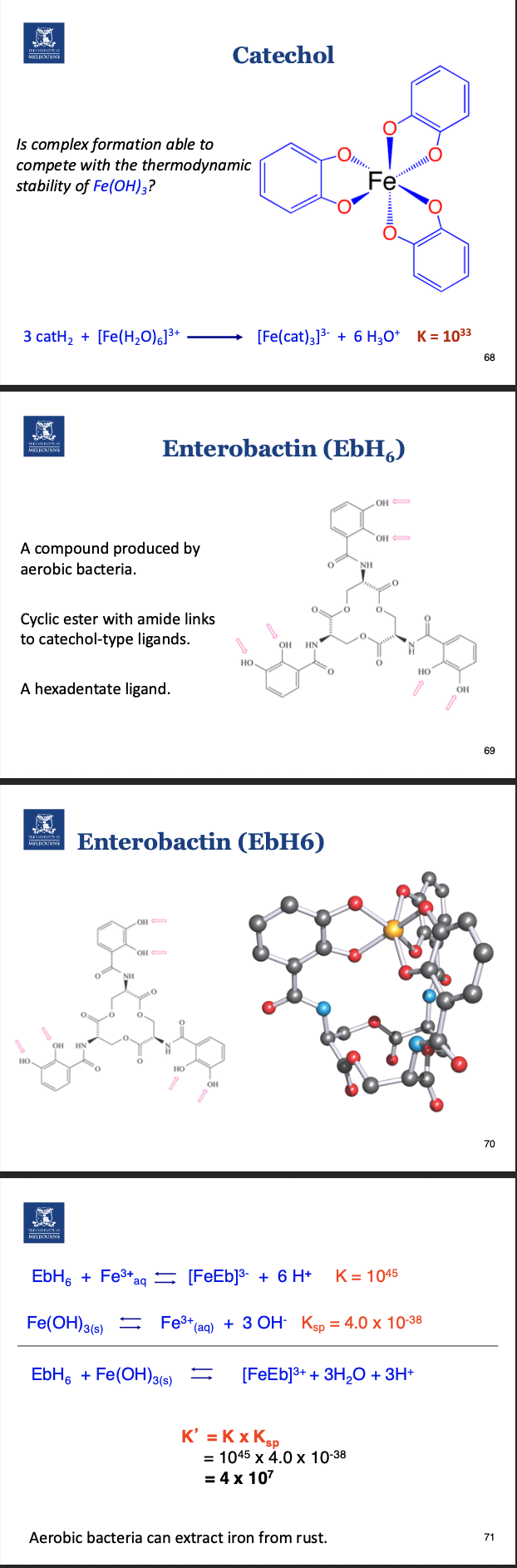
Chelate effect.
Due to the fact that enterodactin has 6 interactions with the iron, it is more stable.
One ligand versus three.
How is iron obtained via nutrition?
Two types of iron: Heme from meat and non-heme iron.
Haem iron, mostly Fe2+ (ferrous), is attached to a porphyrin ring, keeping ferrous iron soluble, protected and ready to absorb.
Non-haem iron, mostly Fe3+ (ferric), must be converted to ferrous iron before being absorbed.
How is iron taken in by the human body?
Transferrin
list more
How is non-heme iron complexed in food?
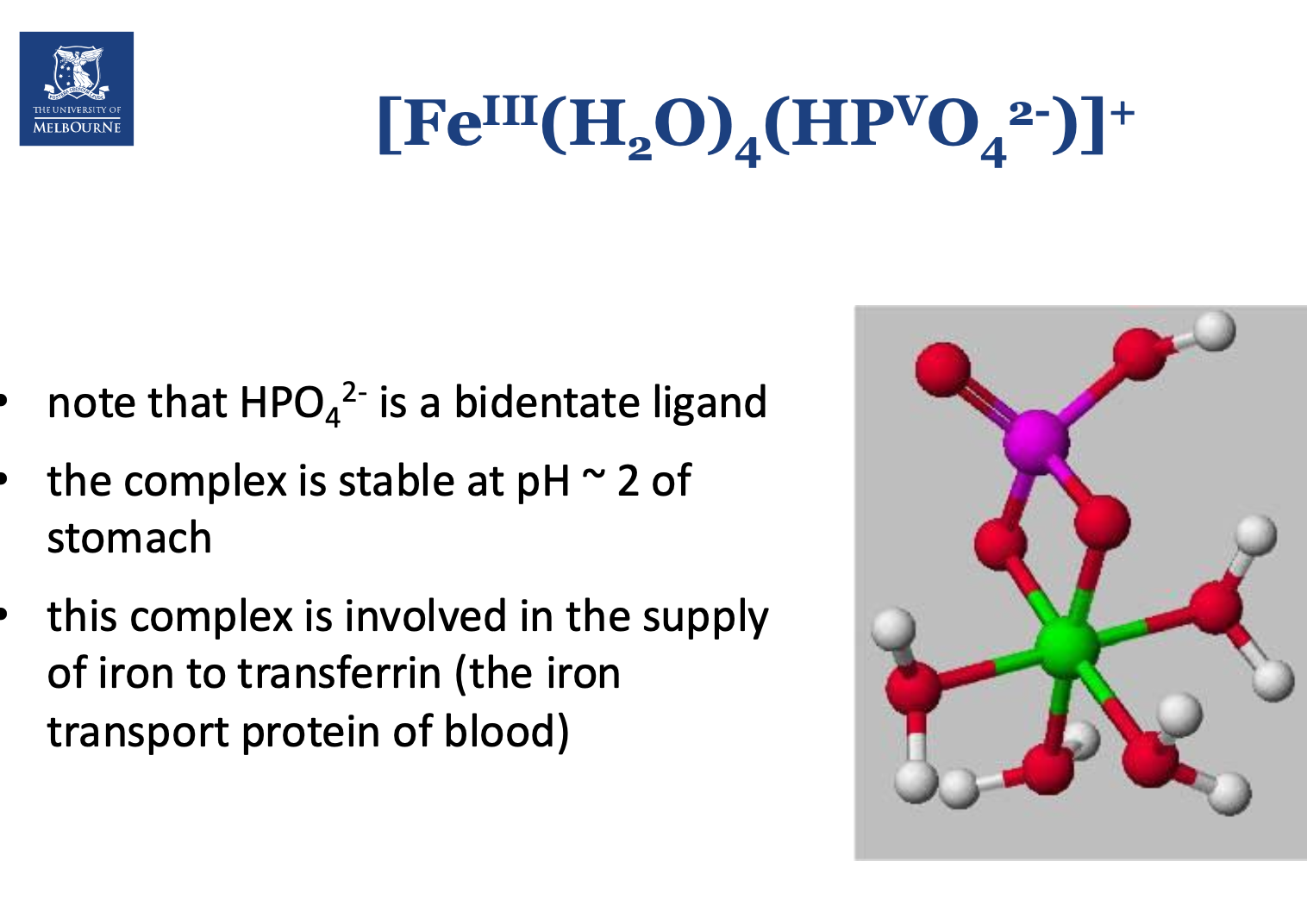
“The bioavailability of iron is limited.” What does this mean?
Not all the iron consumed in the body is absorbed.
Ferric iron is insoluble at around pH 7, thus needs to be converted to ferrous iron.
There are homeostatic mechanisms to ensure that not too much iron is absorbed.
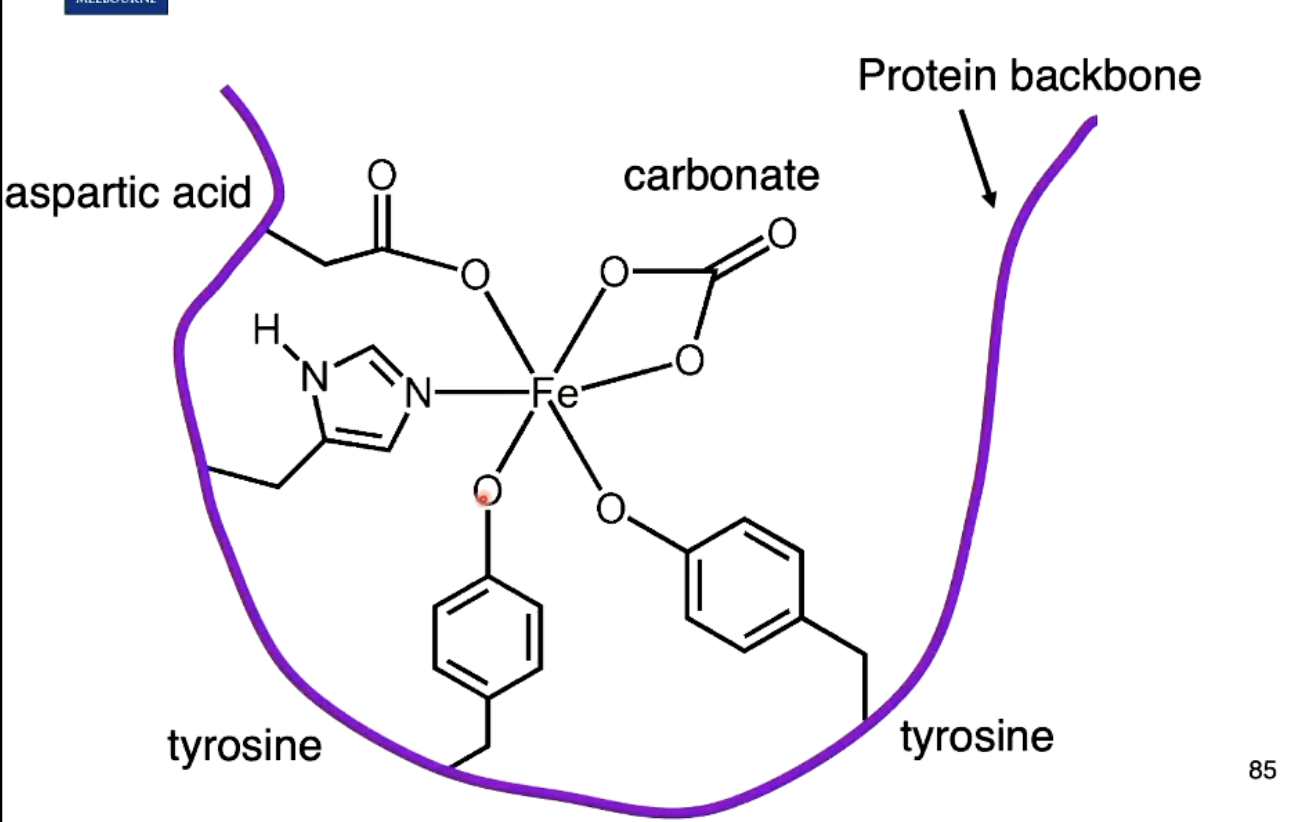
What ligands attach to Fe3+ in transferrin? Draw it.
Octahedral coordination geometry, with coordination number of 6.
Ligands are:
2 tyrosine (deprotenated oxygen atom)
aspartic acid (deprotenated oxygen atom, only monodentate, but in other circumstances may be bidentate)
carbonate (bidentate ligand)
histidine (deprotenated nitrogen atom)
REST OF AA IS THERE, DRAW IT.
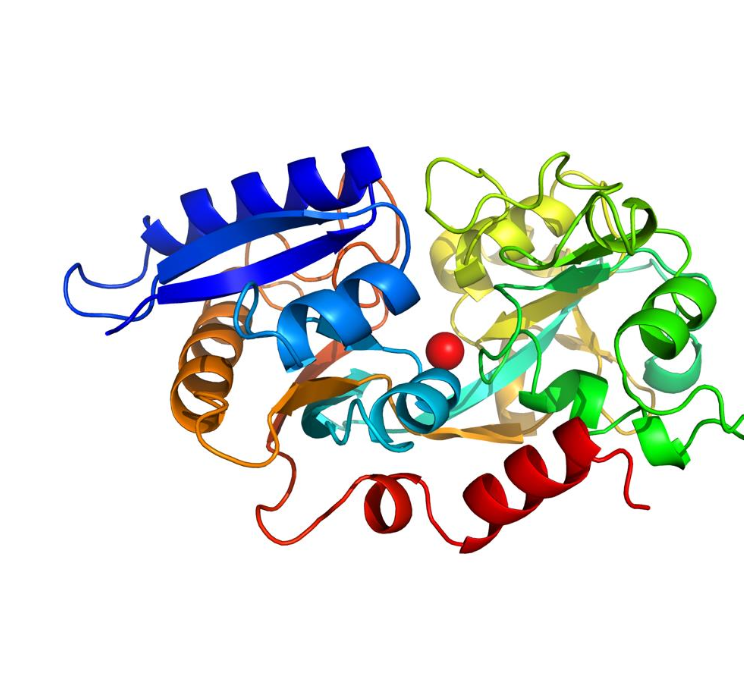
What is the mechanism of transferrin?
Once absorbed, Fe3+ is taken up by the protein transferrin. Note that for iron to be absorbed, it must be in the form of Fe2+, but it is reconverted to Fe3+ for transferrin.
Transferrin transports Fe3+ in the blood to where it is needed.
It brings Fe3+ to the cell via a transferrin receptor. When it goes into the cell, there is a slight drop of pH, causing Fe3+ to be released.
This drop in pH (from around 7 to 5.5), causes the protonation of the histidine residue, making it no longer a ligand, due to the electrons being used on the hydrogen atom now.
The demetalled protein is returned to the outside of the cell, where it begins its search for a new Fe3+ ion.
What does transferrin pass iron to?
Ferritin.
Ferritin posses three key structural features:
-protein coat
-rust core
-core-protein interface.
Ferritin has 24 independent 4 helical bundles (4 alpha helices) that are identical that forms a functional ferritin protein.
Can convert Fe2+ to Fe3+
When iron is required, ferretin releases iron.
What is the function of myoglobin and hemoglobin?
Myoglobin- O2 storage in muscular tissues.
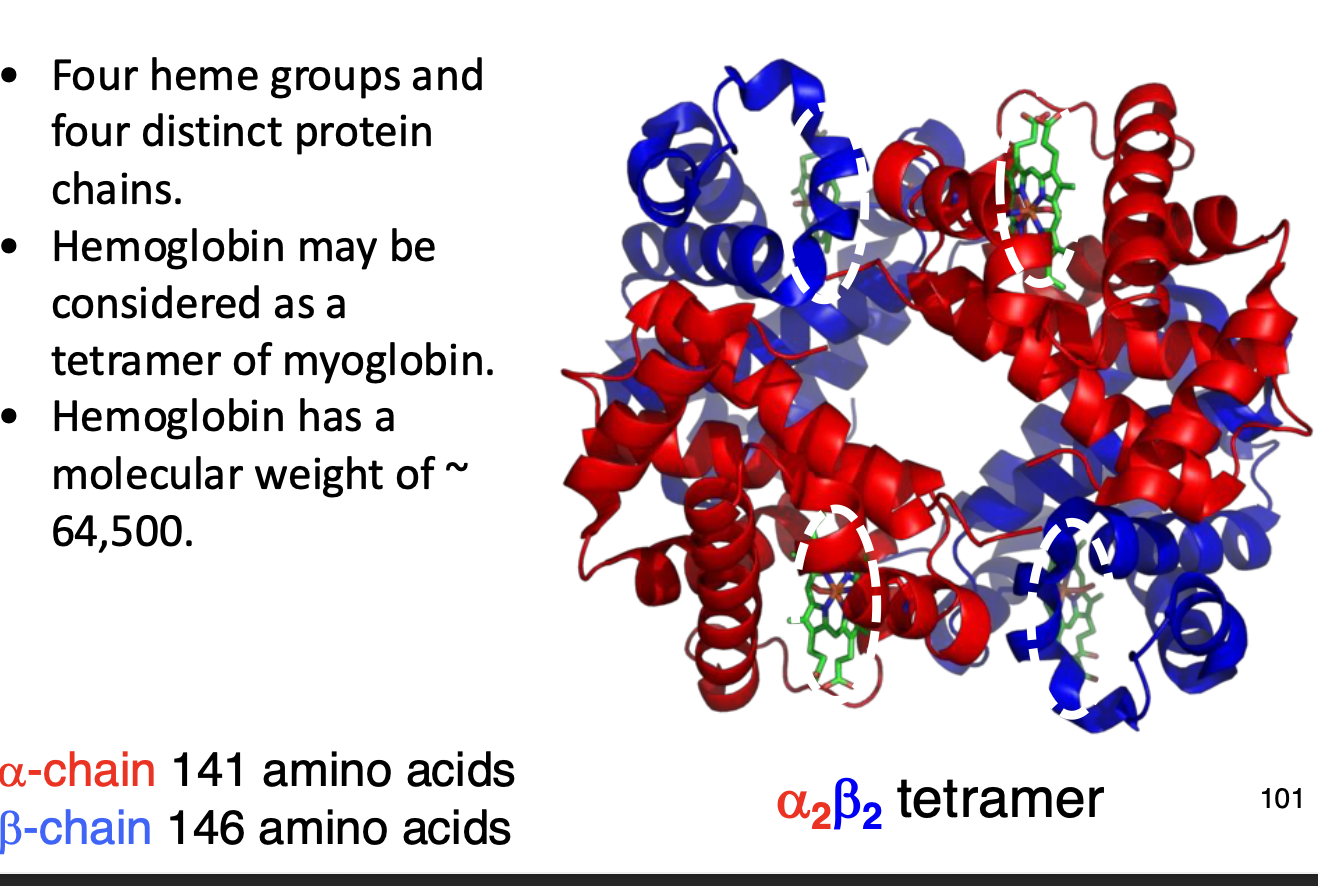
Hemoglobin- O2 transport from lungs. 2 PP chains, ∂ subunit and ß subunit.
A homodimer of a heterodimer which forms a tetramer.
Heterodimer refers to an ∂ chain and ß chain coming together, where due to having two of these heterodimers, it is called a homodimer.

Why do we need haemoglobin and myoglobin?
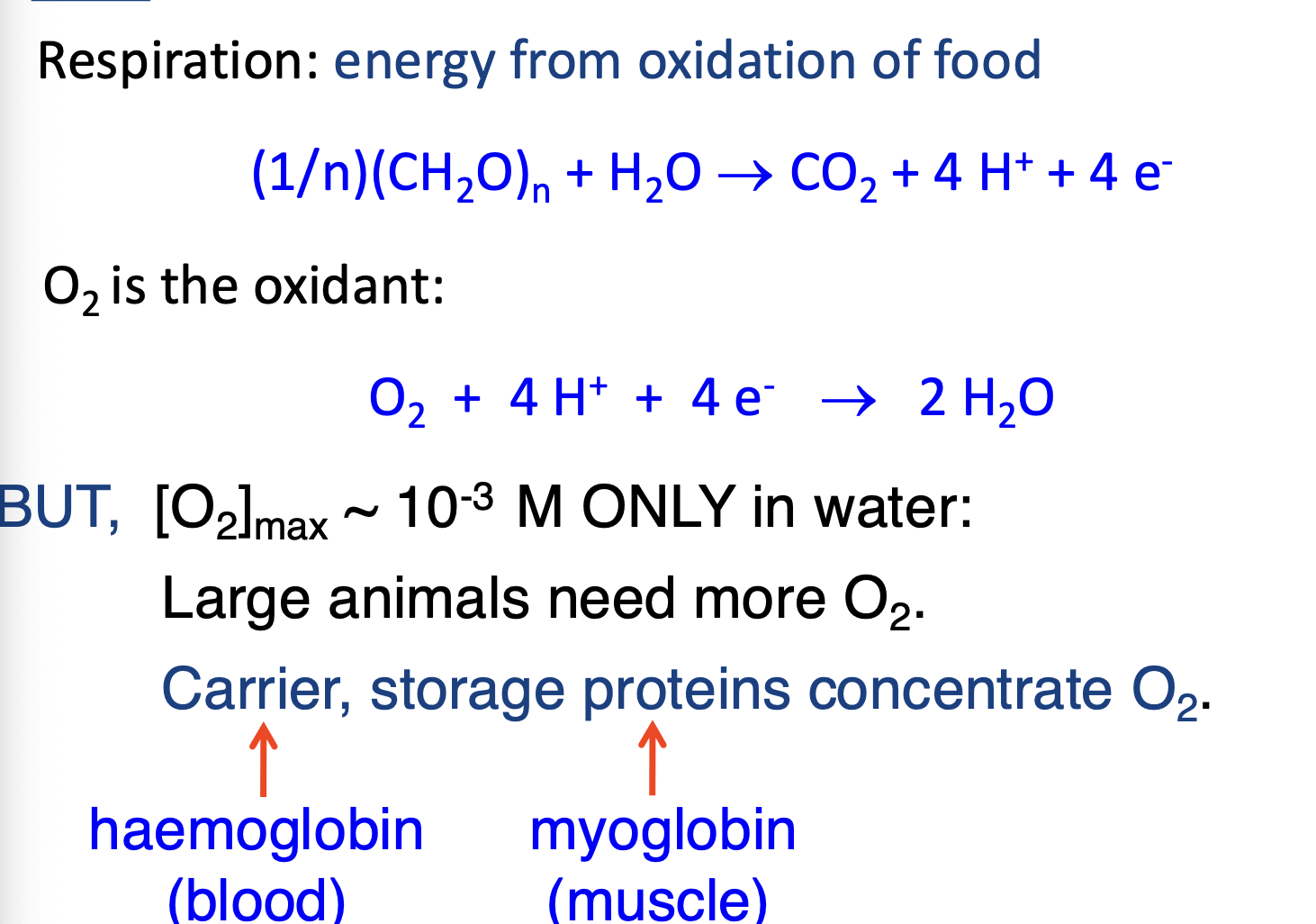
What is the porphyrin ligand?
The PL is a flat unsaturated, conjugated molecule (alternating double and single bonds), which upon deprotonation is able to bind a metal ion at its centre (note the two hydrogen atoms).
Tetradentate ligand (can form 4 coordination bonds).
Porphyrins are hydrophobic, but once coordinated to iron and incorporated into heme proteins (MUST BE), they form functional, bioavailable complexes that do not reduce iron solubility in any problematic way.
It is aromatic and has many resonance forms.
If porphyrin ligand binds to Fe2+ \, it is called Haem.
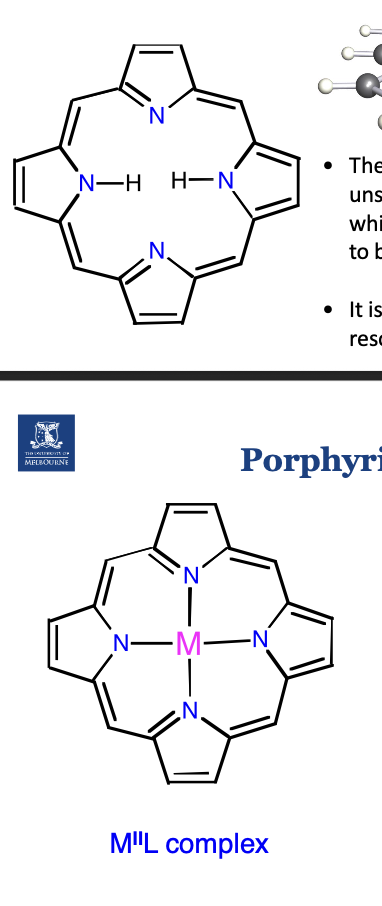
Describe the haem gorup in myoglobin.
Ensures Fe binds in the correct position.
Ensures specificity of Fe.
Ensures Fe2+ stays as Fe2+, and allows oxygen to reversibly bind.
Describe the p[O2] graphs for myoglobin and haemoglobin.
As p[O2] decreases, there is steep decrease in the graph of Hb, this is because more O2 is being released to the muscle to oxygenate the muscle.
As pH decreases in the blood, Hb on graph decreases, because as a muscle is active, producing lactic acid, decreasing pH, releasing O2 into the muscles, for binding via myoglobin.
![<p>As p[O2] decreases, there is steep decrease in the graph of Hb, this is because more O2 is being released to the muscle to oxygenate the muscle.<br><br>As pH decreases in the blood, Hb on graph decreases, because as a muscle is active, producing lactic acid, decreasing pH, releasing O2 into the muscles, for binding via myoglobin.</p>](https://knowt-user-attachments.s3.amazonaws.com/203ddc05-07ac-48cb-ae17-32770afb03b7.png)
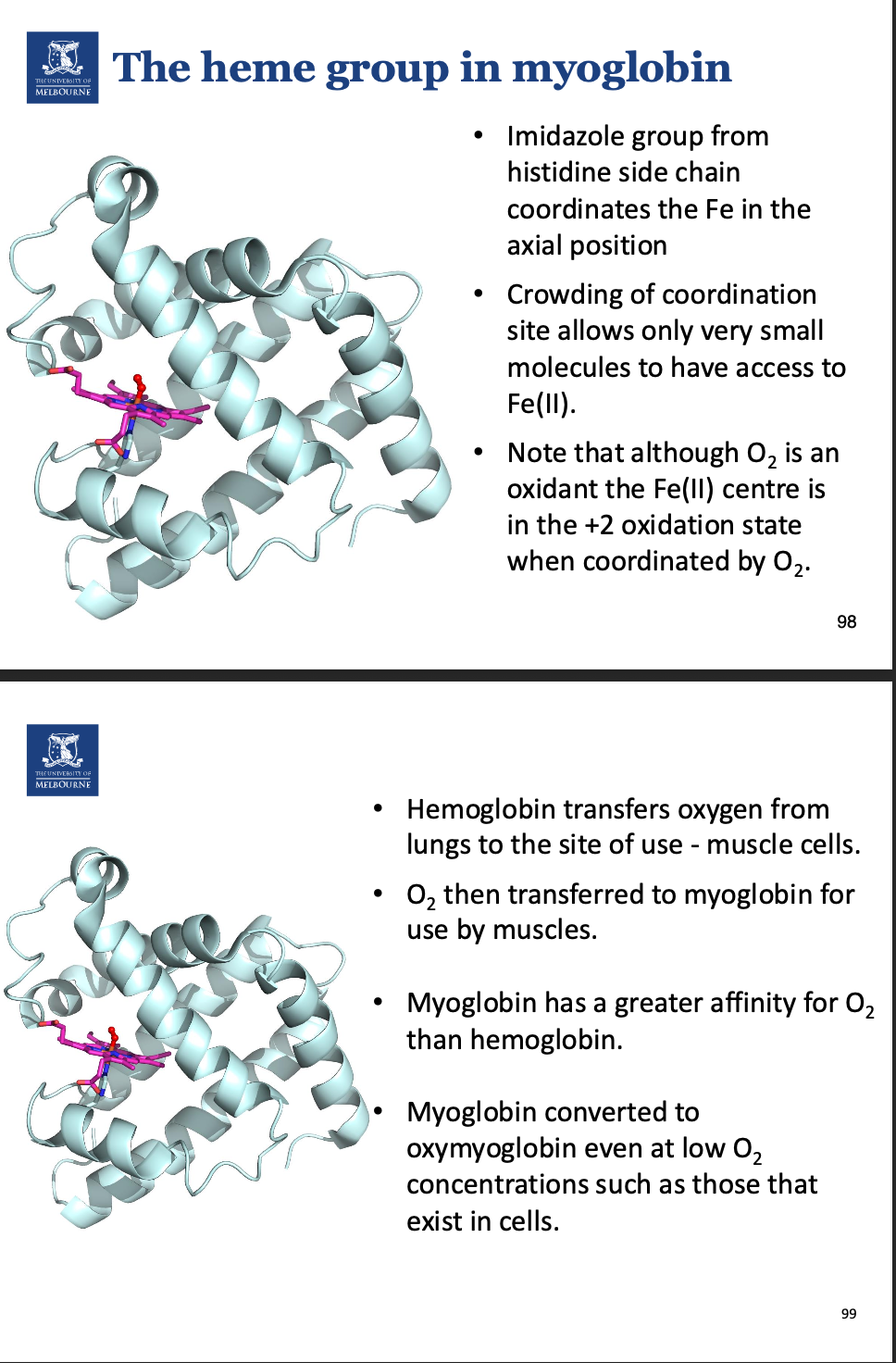
Describe hemoglobin.
All chains can bind to O2.
Describe the cooperativity in hemoglobin.
When O2 binds to haemoglobin, 3 other O2 will bind.
It becomes easier and easier for O2 to bind after each subsequent oxygen. Needed for efficiency.
When O2 dissociated, all O2 dissociates.
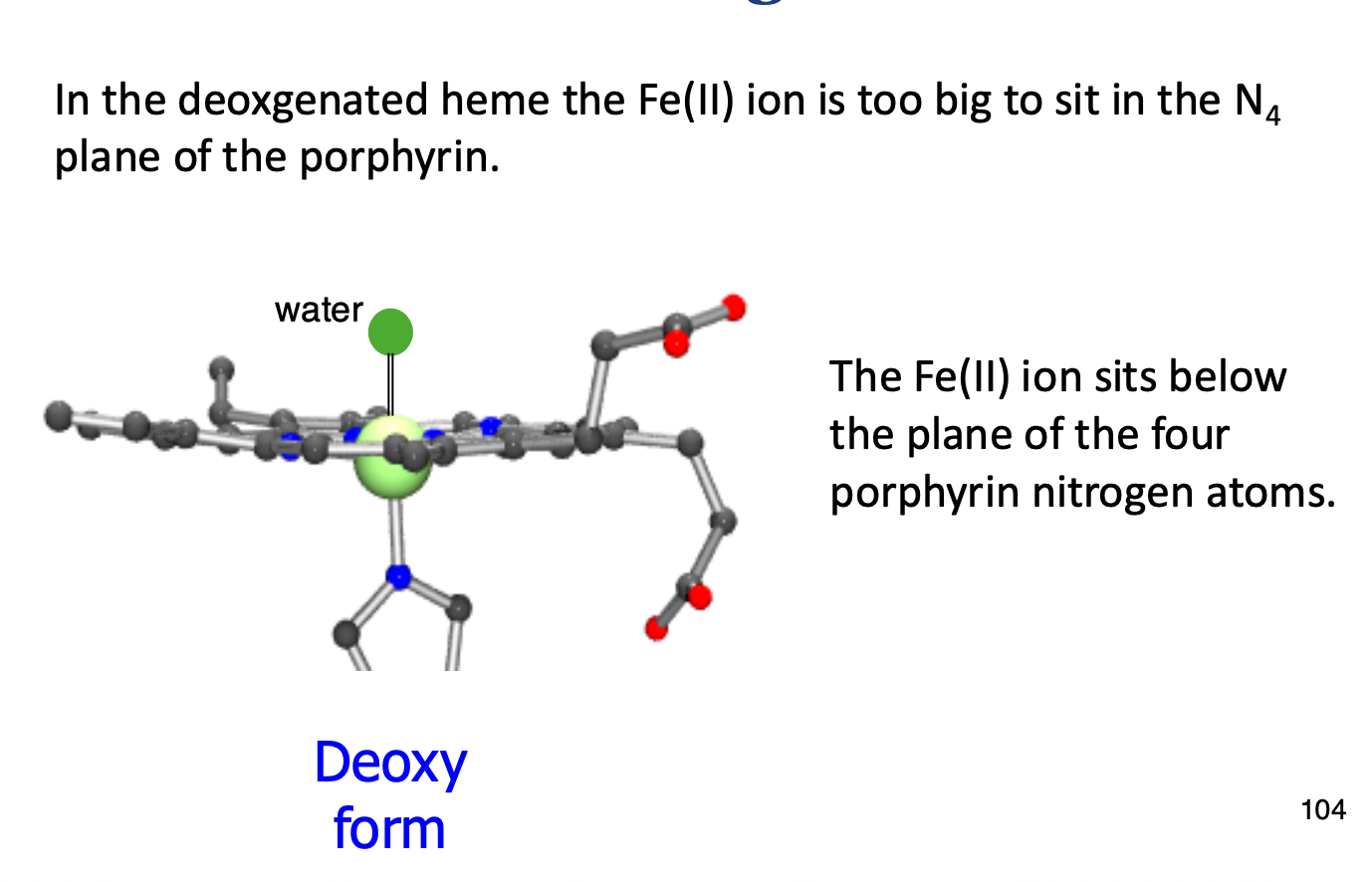
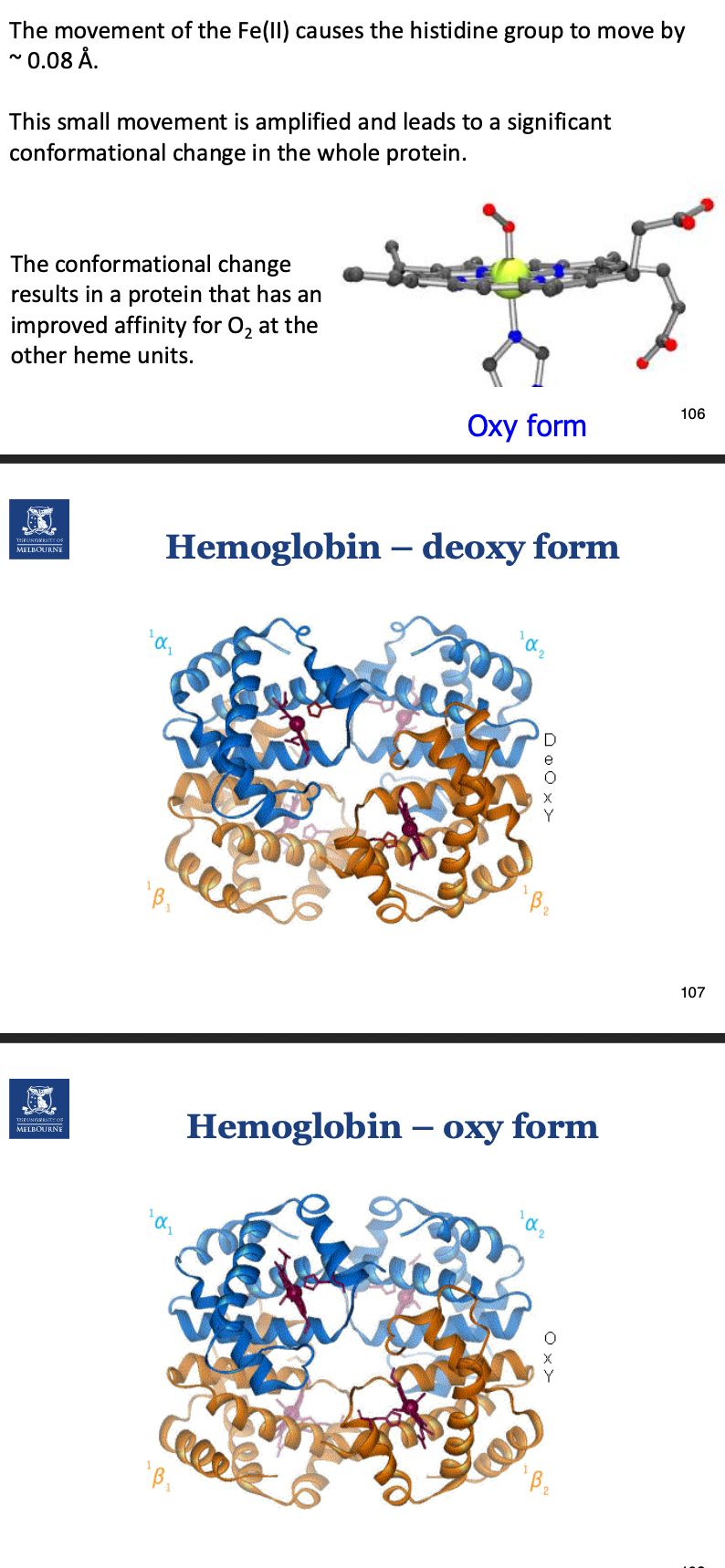
In a deoxygenated Hb, how does Fe2+ bind?
Fe2+ sits out of place on the porphyrin ring as it isn’t being pulled into the plane by O2 binding.
When the porphyrin ring is deoxygenated, it is in a tense state, but once O2 binds it is in a relaxed state.
This relaxed state, along with Fe2+ shrinking (ionic atomic radius) once O2 binds, allows it to sit in allignment with the heme plane.
In oxygenated haem, how does Fe2+ bind?
O2 binds, causing the porphyrin ring to go from tense state to relaxed state, also the ionic atomic radius of Fe2+ shrinks.
This causes the movement of Fe2+ to be in allignment with th eheme plane. This pulls a histidine residue, which is bound to the Fe2+. The histidine is attached to a helix, which also is moved.
Describe the difference in structure between deoxy and oxy form of haemoglobin. Why?
Becomes a bit ‘tighter’ (don’t say that).

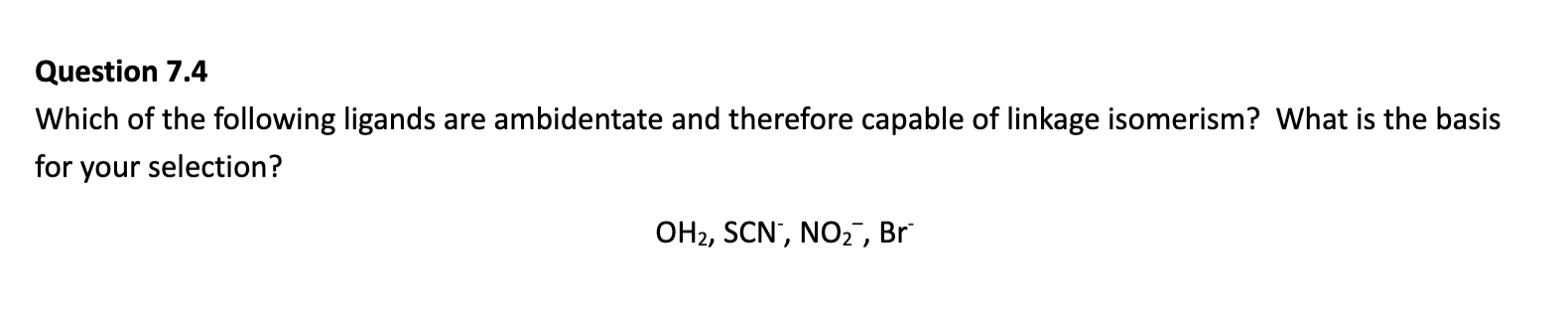
SCN– is an ambidentate ligand as it has 2 possible donor atoms. It can coordinate through the sulfur atom or the
nitrogen atom, but not both at the same time.
NO2– is an ambidentate ligand as it has 2 possible donor atoms. It can coordinate through the nitrogen atom or the
either of the oxygen atoms, but not both at the same time.

[CO(en)2(CO3)2+]+
![<p>[CO(en)<sub>2</sub>(CO<sub>3</sub>)<sup>2+</sup>]+</p>](https://knowt-user-attachments.s3.amazonaws.com/be5d0e94-ca16-4257-bbba-fd2fc85bc6cf.jpg)
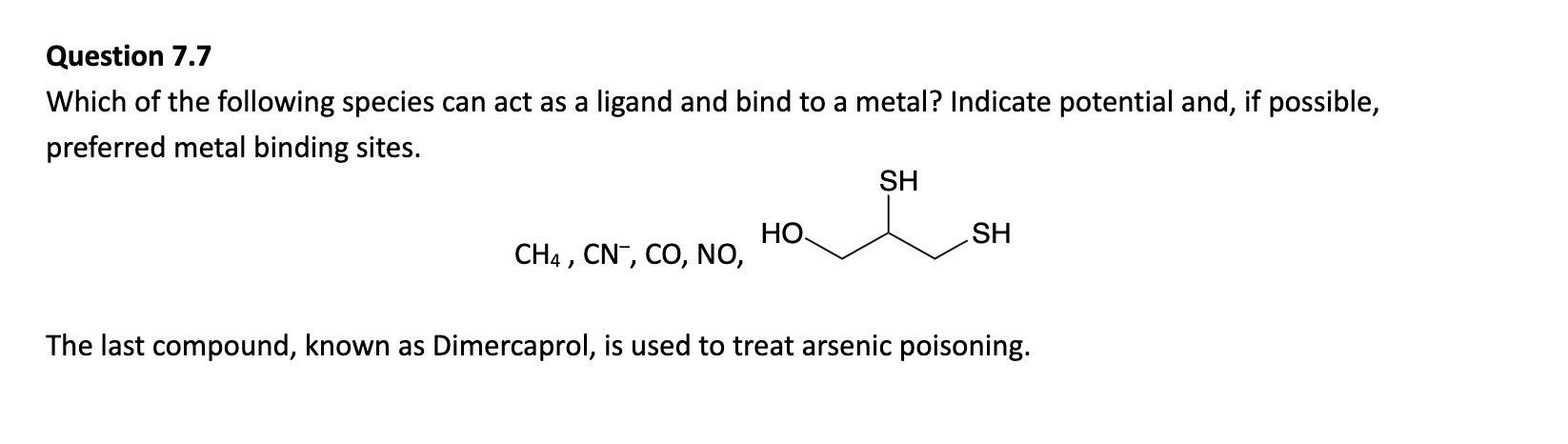
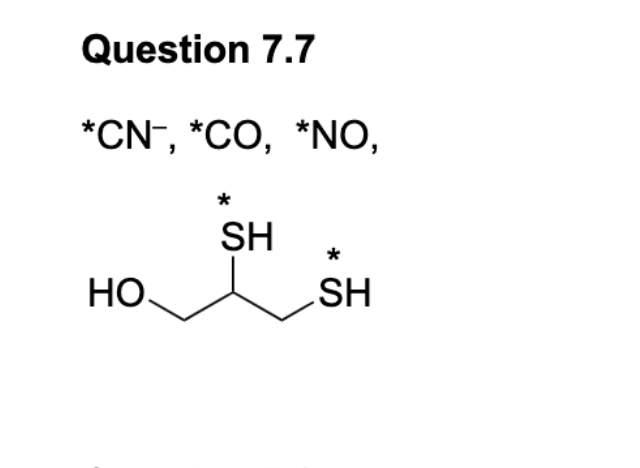
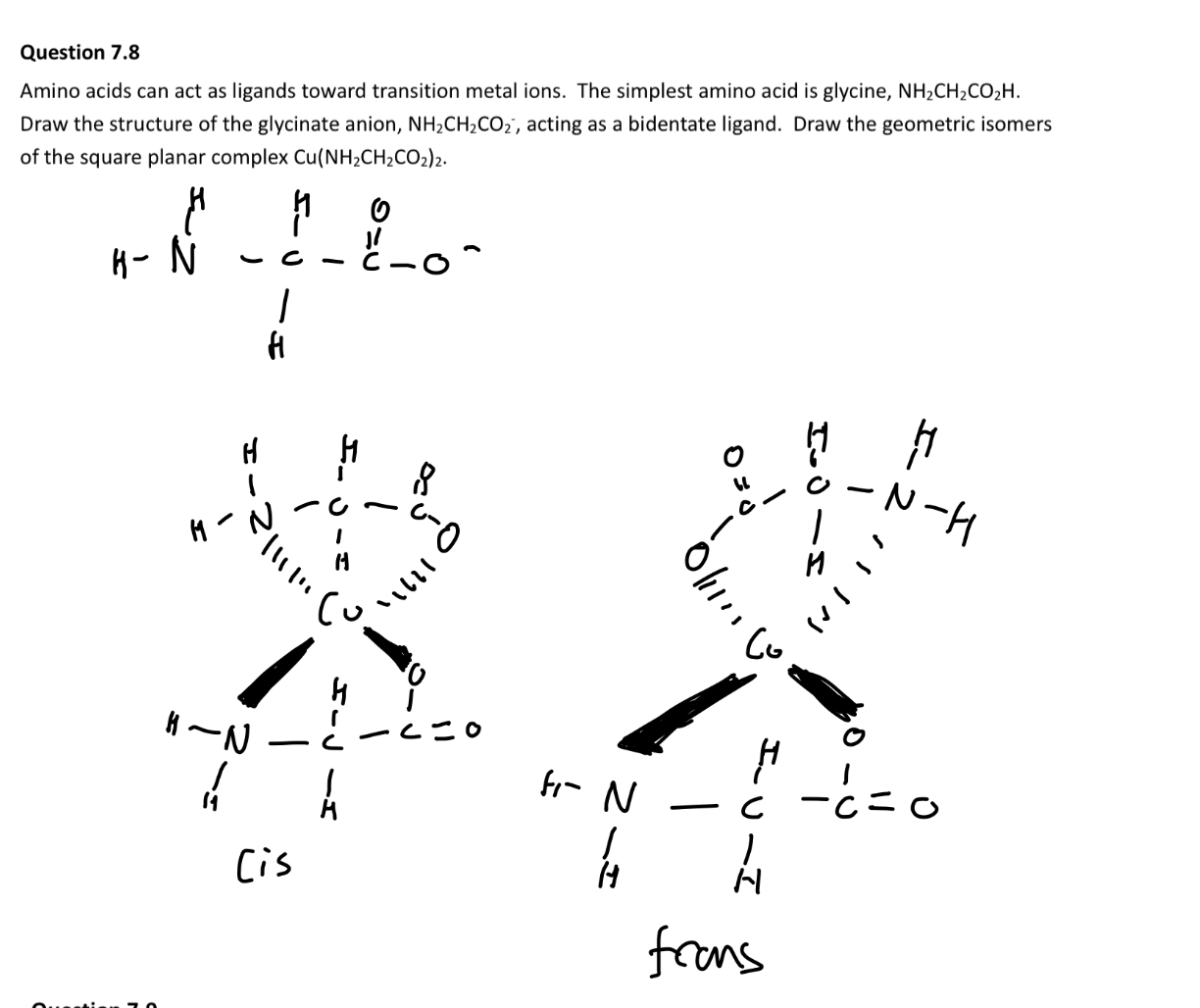


Look at double bonds.
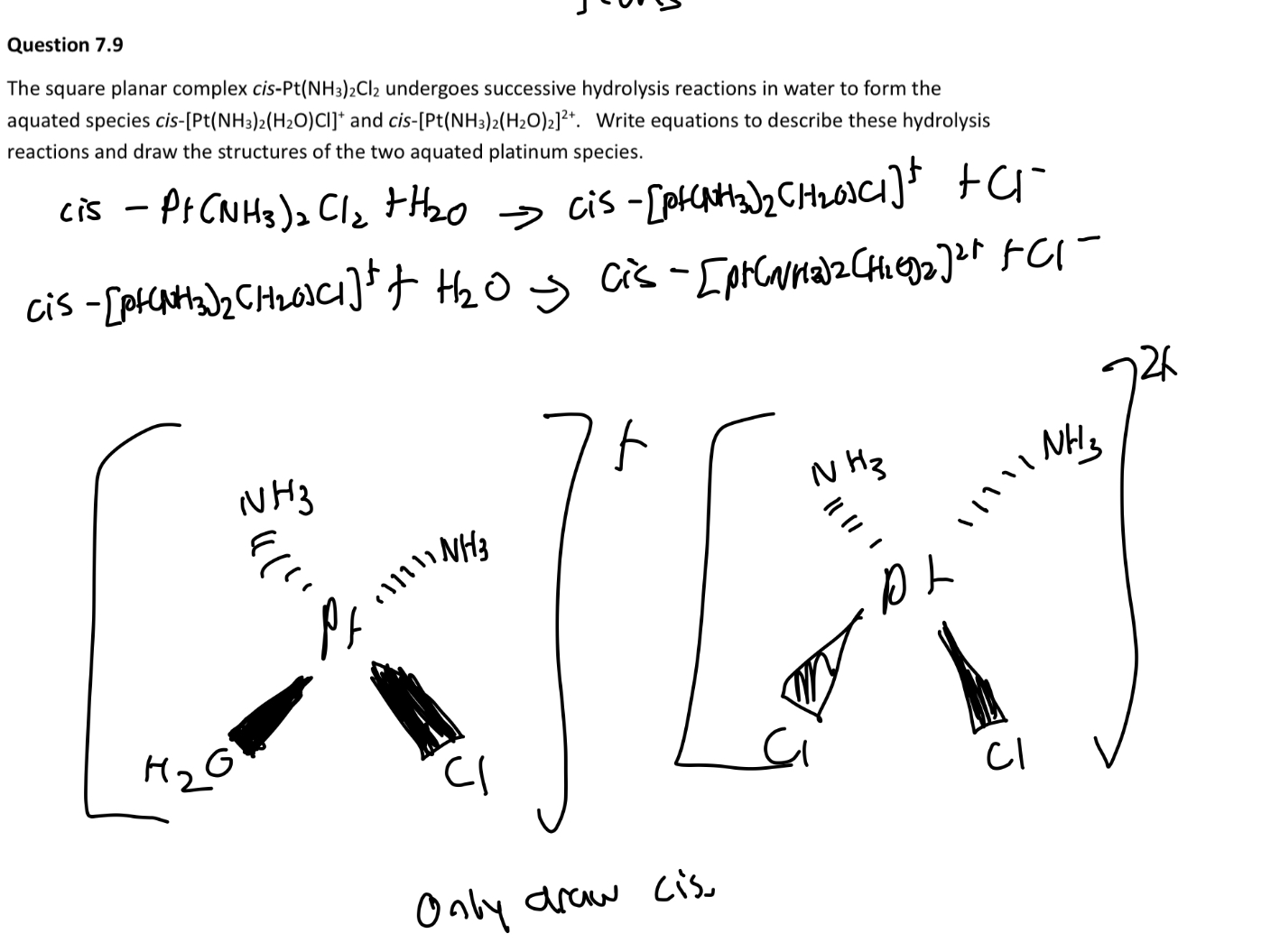
Why can’t carbonate be a tridentate ligand?
Due to the orientation of the molecule, it isn’t possible for all oxygens to form coordination bonds.
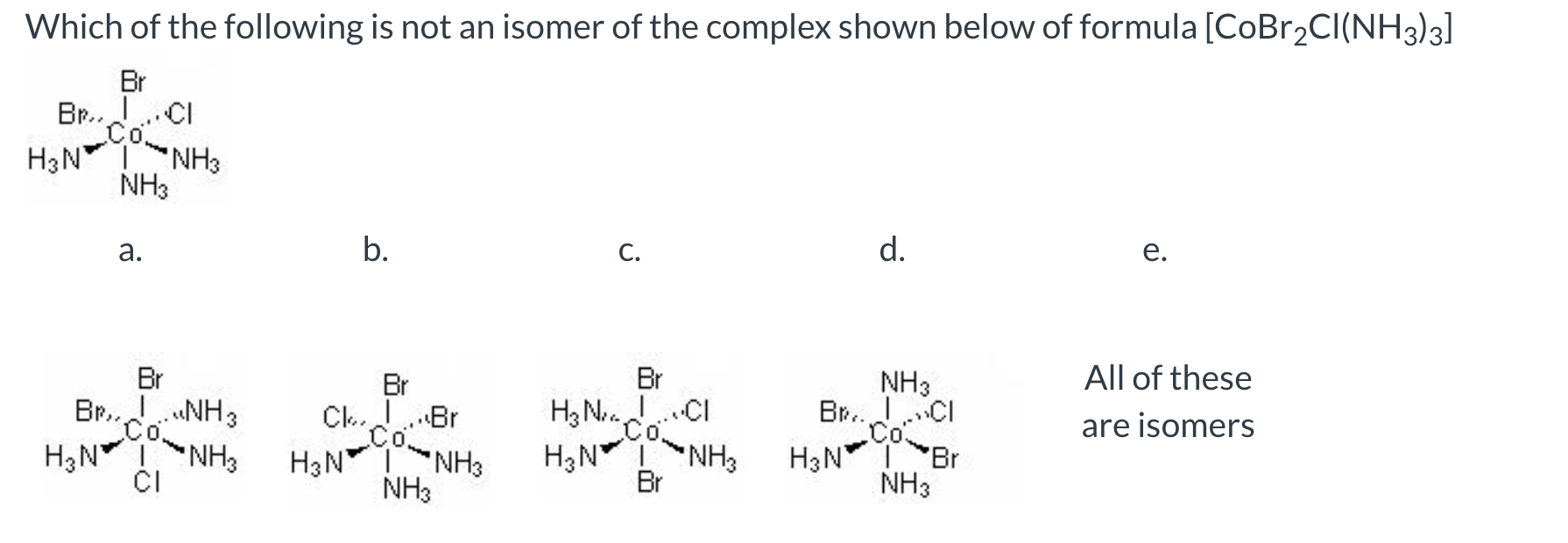
B.
B is the same as the top image.
Draw en, enterobactin and catechol.
O2 binds to haemoglobin in a cooperative process. Explain this statement. Would you expect myoglobin to exhibit
similar behaviour?
Hemoglobin (Hb) has four subunits, each with a heme group that can bind one O₂ molecule.
Cooperative binding means that the binding of O₂ to one subunit increases the affinity of the remaining subunits for O₂
This is because the shifting in structure makes O2 easier to bind.
This would not be exhibited in myoglobin, as myoglobin is a monomeric protein, thus can only bind to one O2.