Biochem Lec 28/29- Oxidative Phosphorylation II/III: The ETC and ATP Synthase
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
Why are the intermembrane space and the matrix side referred to as the P and N sides, respectively?
Intermembrane Space→ P side→ protons are pumped to this side giving it a more positive charge
Matrix Side→ N side→ protons are pumped out of this space giving it a more negative charge
Briefly describe the ETC including how it relates to the citric acid cycle, mobile carriers, and electron flow.
Site of electron flow from NADH and FADH2 (succinate) from the CAC and other catabolic pathways
Four multienzyme complexes connected by two mobile carries: ubiquinone (Q) and cytochrome c
Electrons flow from –Eo’ to +Eo’ (delta Eo’ is positive, so delta Gº’ is negative)
During electron flow, H+ are pumped from the matrix to the cytosolic side of the mitochondrial inner membrane
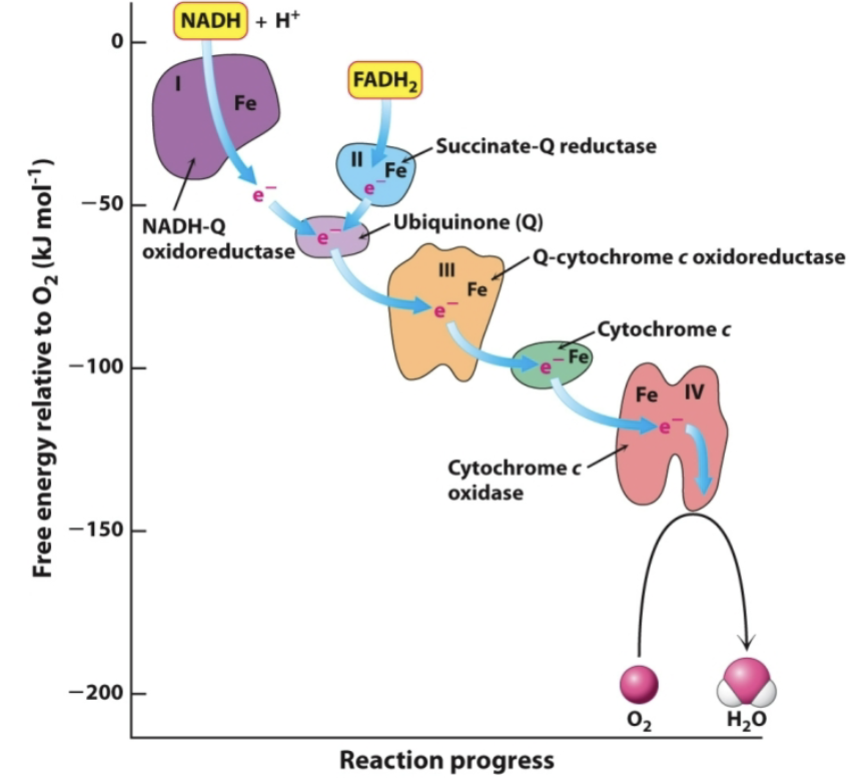
What are the large enzyme complexes and what are their metal/coenzyme based prosthetic groups? What do they do?
Four large enzyme complexes: I, II, III, IV
Various metal and coenzyme based prosthetic groups that carry electrons:
Flavin coenzymes (FAD and FMN)→ I and II
Iron-sulfur proteins (Fe-S)→ I, II, and III
Heme groups→ III and IV
Copper centers→ IV
Coenzyme Q and Cytochrome C shuttle electrons between complexes

Complex I: name and prosthetic group
Name→ NADH-Q oxidoreductase
Prosthetic group→ FMN and Fe-S
Complex II: name and prosthetic group
Name→ Succinate-Q reductase
Prosthetic group→ FAD and Fe-S
Complex III: name and prosthetic group
Name→ Q-cytochrome c oxidoreductase
Prosthetic group→ Heme bH, bL, c1, and Fe-S
Complex IV: name and prosthetic group
Name→ Cytochrome c oxidase
Prosthetic group→ Heme a, a3, CuA, and CuB
Describe the pathway of NADH and succinate.

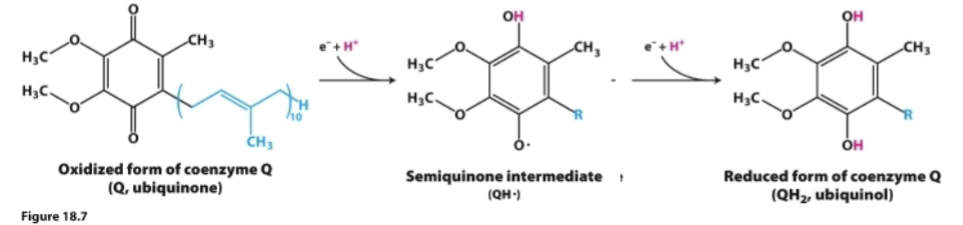
Describe ubiquinone. How does it behave, how many states of oxidation does it have, and what does it do?
Mobile carrier
Highly hydrophobic and freely diffuses in mitochondrial inner membrane
Can exist in three states of oxidation:
Q Ubiquinone→ most oxidized
QH● semiquinone→ radical species after accepting one e- and 1 H+
QH2 Ubiquinol→ most reduced after accepting two e- and 2 H+
Connects Complex I and II with complex III
Describe Cytochrome C. What does it do and what does it involve?
Mobile carrier
Small peripheral membrane protein on intermembrane/cytosolic side of mitochondrial membrane
Heme group with a single Fe atom that can carry one electron (Fe3+ or Fe2+)
Accepts electrons from QH2 and donates them to O2
Connects complex III with IV

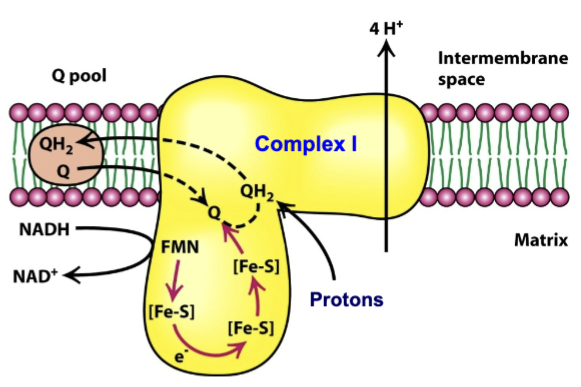
What is Complex I and what does it do/involve?
Complex I: NADH-Q oxidoreductase
Huge protein complex (46 polypeptides)
Two types of tightly bound prosthetic groups:
FMN→ similar to FAD but lacking ADP portion
Iron sulfur (FeS) clusters→ nonheme iron proteins that carry one electron
During transport of 2 electrons, 4 H+ pumped into the cytosolic side of the inner membrane
What is the net reaction of Complex I?

What is Complex II and what does it do/involve?
Complex II: Succinate-Q reductase
Contains the succinate dehydrogenase activity from the citric acid cycle
Electrons flow directly from succinate to a covalently bound FAD
Iron sulfur proteins transfer electrons from FADH2 to Q
No protons are pumped by complex II
What is the net reaction of Complex II?

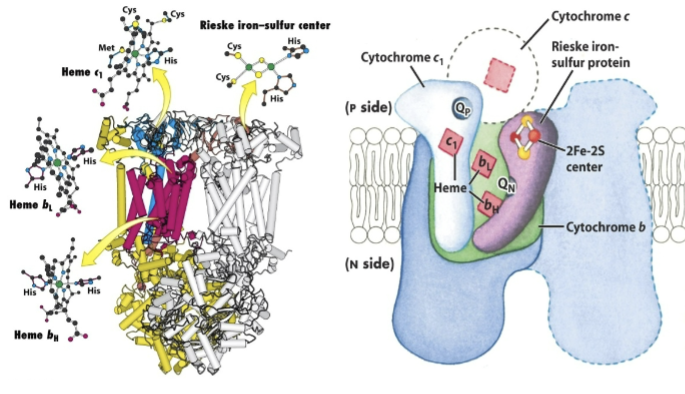
What is Complex III and what does it do/involve?
Complex III: Q-cytochrome c oxidoreductase
Four tightly bound prosthetic groups:
Cytochrome b→ contains two heme groups bL and bH
Reiske Iron sulfur cluster
Cytochrome c1→ delivers electrons one at a time to cytochrome c and has one heme group c1
During electron flow, 2 H+ are released from QH2 and 2 H+ are additionally pumped from the matrix to the cytosolic side
What is the net reaction of Complex III?

What is Complex IV and what does it do/involve?
Complex IV: cytochrome c oxidase
Transfers electrons from cytochrome c to O2
Pumps protons across the membrane in the process
Contains a core of 3 subunits that contain intermediate electron donors
What is the net reaction of complex IV?

Briefly describe the mechanism of complex IV.
Cytochrome c oxidase contains a core of three subunits that contain intermediate electron donors:
Two hemes: heme a and a3
2 “copper centers”: Cu A and Cu B
Electrons flow from Cytochrome C to CuA and then are transferred to heme a→ heme a3→ CuB
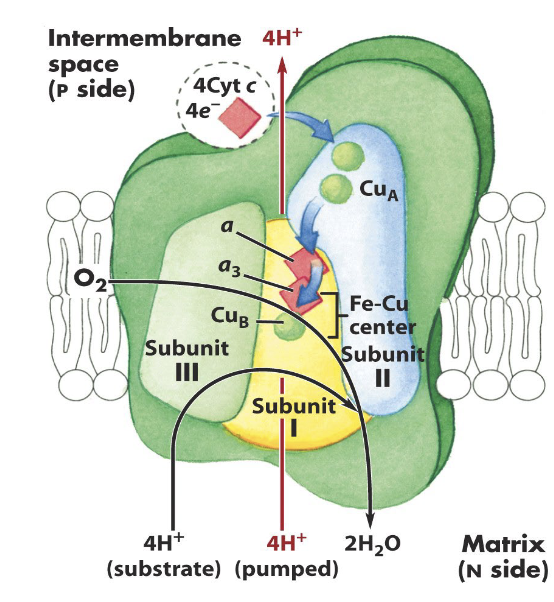
What is the issue with the mechanism of complex IV?
The oxygen intermediates are reactive:
Aided by the binding of oxygen to both copper and the iron of the heme→ forms peroxide bridge
Complex IV must hold a molecule of molecular oxygen until 4e- are delivered from 4 cytochrome c molecules
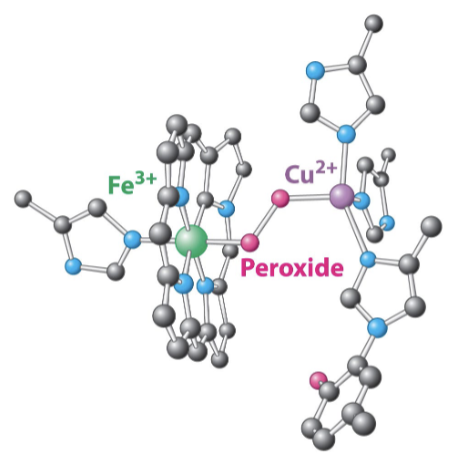
Describe the mechanism of complex IV in terms of electron flow.
Two cytochrome c molecules deliver electrons to CuB and heme a3
Oxygen binds and forms a bridge between CuB and heme a 3
Two more cytochrome c molecules reduce the bound oxygens to hydroxyl groups
Protons cleave the hydroxyl groups and release water
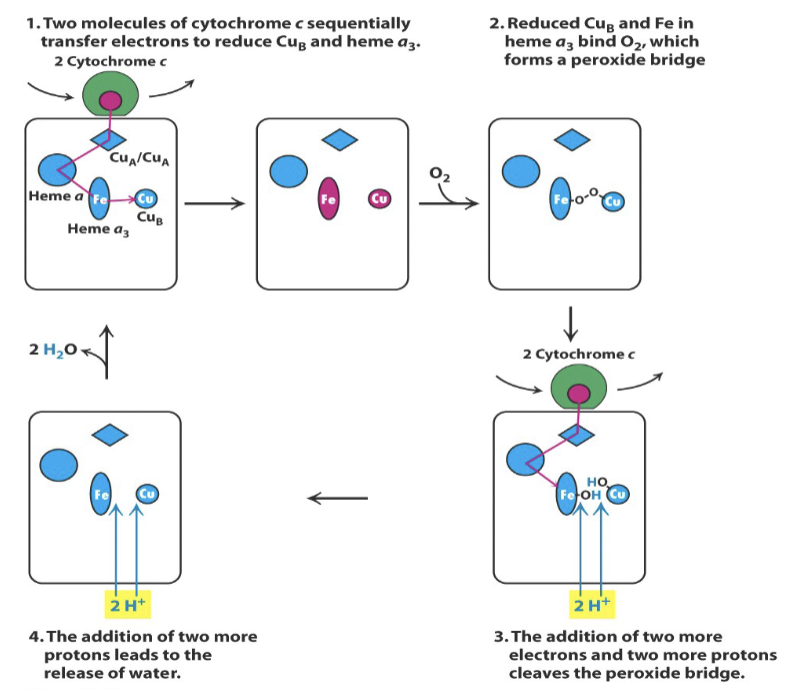
Image depicting overall mechanism of complex IV
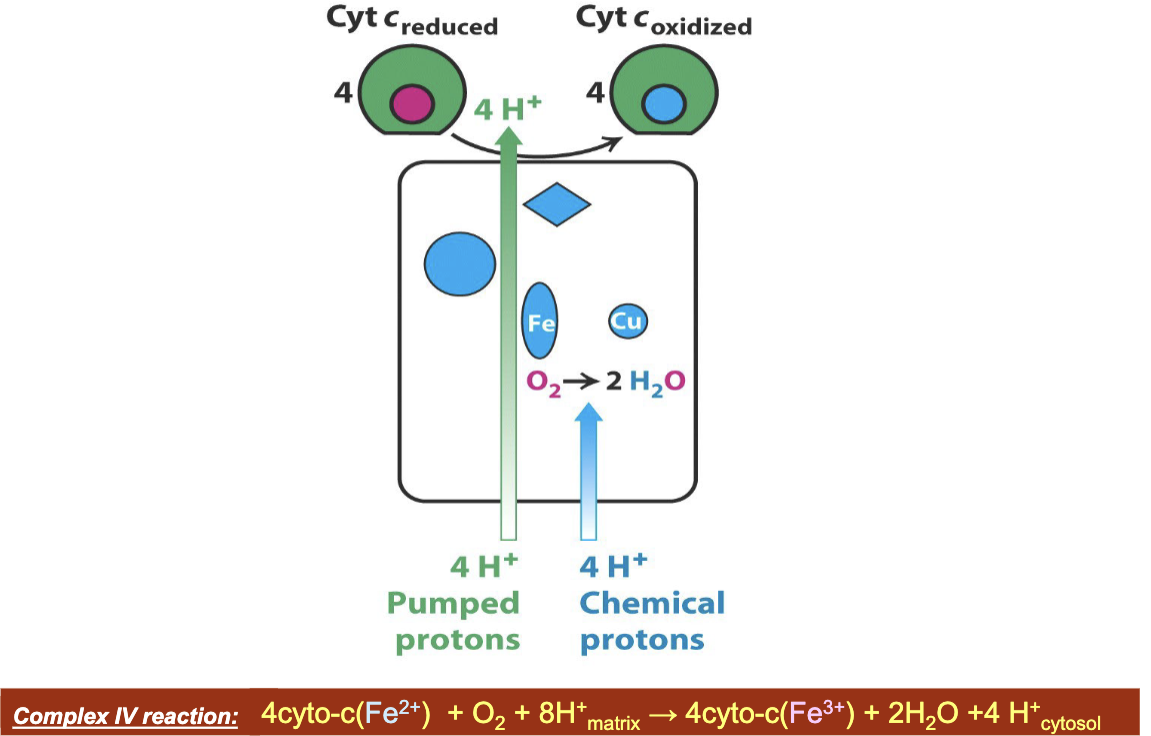
What are the reactions using both NADH and succinate by which the ETC converts reducing power to an H+ gradient?
NADH: NADH + H + ½ O2→ NAD+ + H2O
10 H+ pumped to intermembrane/cytosol per 2 e-
Succinate: Succinate + ½ O2→ Fumarate + H2O
6 H+ pumped to intermembrane/cytosol per 2 e-
Why is electron transport energy stored as a “proton motive force”?
H+ movement by the ETC generates an electrochemical gradient
As we learned before there are two energetic components of this gradient:
A chemical gradient (Δ[H+] a.k.a ΔpH)
An electrical gradient (ΔV )
The energy available in this gradient (ΔG) is referred to as the “proton motive force”
This force powers ATP synthesis by ATP synthase.
![<ul><li><p>H+ movement by the ETC generates an electrochemical gradient</p></li><li><p>As we learned before there are two energetic components of this gradient:</p></li></ul><ol><li><p>A chemical gradient (Δ[H+] a.k.a ΔpH)</p></li><li><p>An electrical gradient (ΔV )</p></li></ol><ul><li><p>The energy available in this gradient (ΔG) is referred to as the “proton motive force”</p></li><li><p>This force powers ATP synthesis by ATP synthase.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/0f7cb82f-d1ef-45bd-ab0e-858bbf0bec61.png)
What is the typical PMF in respiring mitochondria and what does this indicate?
In respiring mitochondria, the PMF is typically 21 kJ/H+
Thus, for NADH moving through the e- transport chain (10 H+), 210 kJ/mol of energy stored
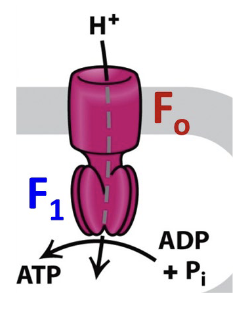
Describe the structure of ATP Synthase (what subunits are present and where are they located?)
ATP synthase: Composed of two multi-subunit components: Fo and F1
Fo is an integral membrane component imbedded in the mitochondrial inner membrane
H+ move through the Fo component from the cytosolic/intermembrane space to the matrix side
The F1 component projects into the mitochondrial matrix. It synthesizes ATP from ADP and Pi
Fo rotates and the F1 is stationary
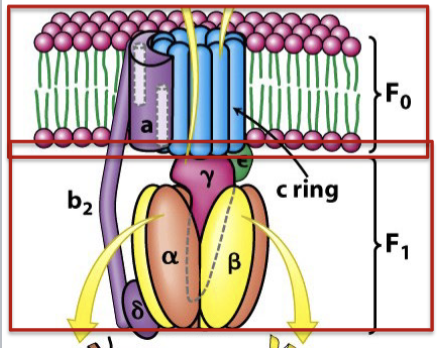
Describe the structure of the components of ATP Synthase
Fo: Integral membrane proton channel:
ring of 10-14 “c-subunits” which form proton channel
”b and a” subunits which connect c-ring to δ subunit of F 1
F1 : Peripheral membrane component that faces matrix and synthesizes ATP
three α and three β subunits form a ring
β subunits synthesize ATP
α and β ring is connected to the γε stalk which bind the c-ring of Fo
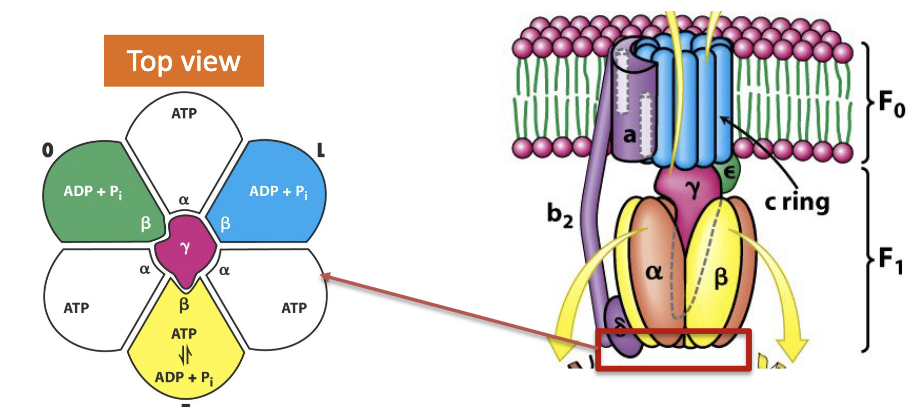
Describe the binding change mechanism of ATP Synthase
Binding change model for H+ coupled ATP synthesis:
ATP synthase is a small “engine”. It has moving and stationary parts:
c-subunits and γε stalk rotate in response to proton movement. These form the “rotor”
The remainder of the structure is stationary. These form the “stator”
Describe the three conformations that can be adopted by ATP synthase.
The γ subunit rotates and interacts with all three β subunits causing them to adapt one of three conformations:
Loose (L): Binds ADP and Pi and traps them in active site
Tight (T): Binds very strongly to ATP (drives ATP synthesis by binding energy)
Open(O): lowest affinity, releases bound ATP
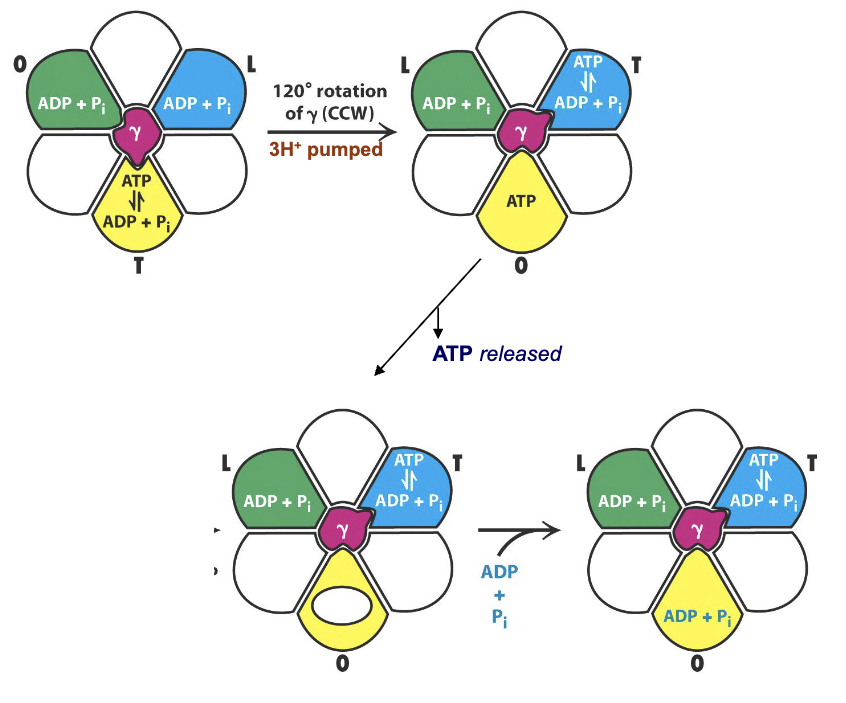
What drives the 120º rotation of the y subunit?
3H+ moving through the c-ring into matrix drives 120o rotation of the γ subunit:
This causes a coordinated change in the conformation of the three β subunits
T →O: synthesized ATP is released
L → T: ADP + P i is converted to ATP
O→ L: ADP + P i is trapped in active site
Describe the structure of Fo.
Proton movement through the Fo involves the c subunits and the a subunit:
Each c-subunit is a two helix integral membrane protein with an Asp residue that carries protons
The a subunit contains two “half channels” through which protons move.
One half channel faces the cytosolic side, protons enter here
Other half channel faces the matrix side, protons exit here
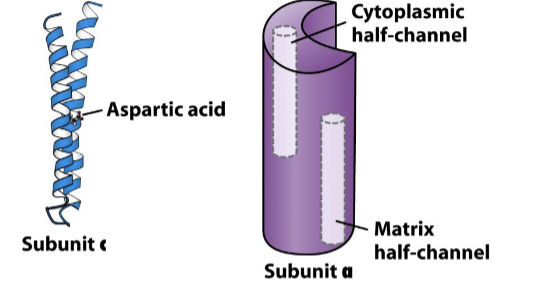
Describe how H+ drives the rotation of the c-ring.
Protons from cytoplasmic/intermembrane side enter one half channel and protonate the asp residue of one c subunit
Simultaneously a proton is released into the matrix from the asp residue of the adjacent subunit

Overall, describe how ATP is synthesized through ATP synthase.
The movement of protons through the half-channels from the cytosolic intermembrane space to the matrix powers the rotation of the c ring
The c ring is tightly linked to the γε stalk, rotating the γ subunit in turn, thus promoting ATP synthesis via the binding change mechanism
For a ring of 10 c subunits, ~3 H+ pumped per 120º rotation needed to synthesize and release 1 ATP
How is ATP transported to the cytosol and what is the net cost?
Once synthesized, ATP needs to be transported to the cytosol while spent ADP needs to enter the mitochondrial matrix
This exchange is performed by an antiport transporter, the ATP-ADP translocase
Since ADP (-3) has one less charge than ATP (-4), this transport costs 1H + equivalent in proton motive force
NET COST:
3 H+ to synthesize an ATP
1 H+ to transport an ATP to cytosol
ADP3-cytoplasm + ATP4-matrix → ADP3-matrix + ATP4-cytoplasm

Oxidative phosphorylation: Net synthesis
NADH + H + ½ O2→ NAD+ + H2O
10 H+ pumped to intermembrane/cytosol side per 2 e-
Succinate + ½ O2→ Fumarate + H2O
6 H+ pumped to intermembrane/cytosol side per 2 e-
Net cost for synthesis and transport of 1 ATP is 4 H+
3 H+ to synthesize an ATP via ATP synthase
1 H+ to transport ATP to cytosol via ATP via translocase
NADH→ 10 H+→ 2.5 ATP
Succinate→ 6 H+→ 1.5 ATP