alkanes and cycloalkanes conformations and cis-trans stereoisomers
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
most stable conformation of ethane
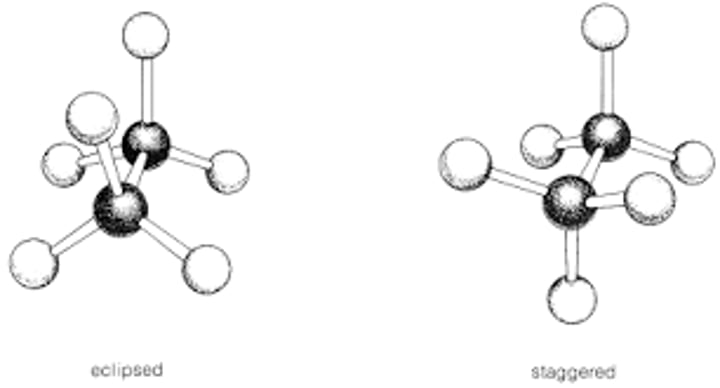
staggered conformaton is more stable than eclipsed- each C-H bond biscts an H-C-H bond
eclipsed is less stable due to electron-electron repulsion in aligned bonds- each C-H bond is aligned with an adjactent C-H bond
staggered is stable because of better electron delocalization
wedge and dash
lines lie in the plane
dashes lie in the back
wedges are coming out of the plane
conformation
the most stable conformer has the lowest energy
any time there is an eclipse in an atom it is high in energy making it very unstable
eclipsed conformers are less stable because of their steric effects- bulkiness (steric hinderance)
torsion angles
eclipsed = 0
gauche- 60
anti- 180
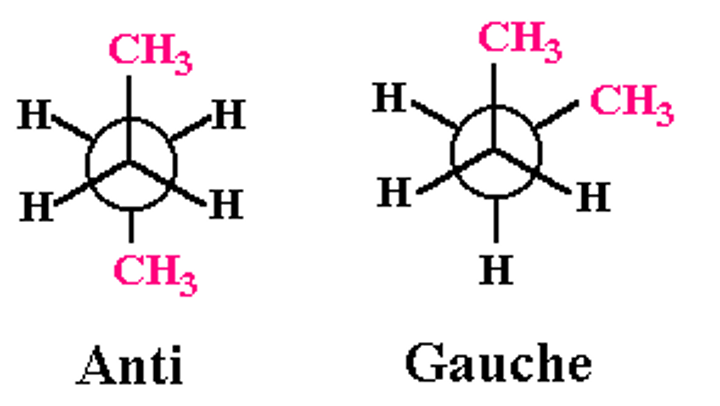
staggered conformations of butane
they are not equivalent
anti conformation is MORE STABLE than gauche
gauche is less stable because of Vanderwall strain involving methyl groups
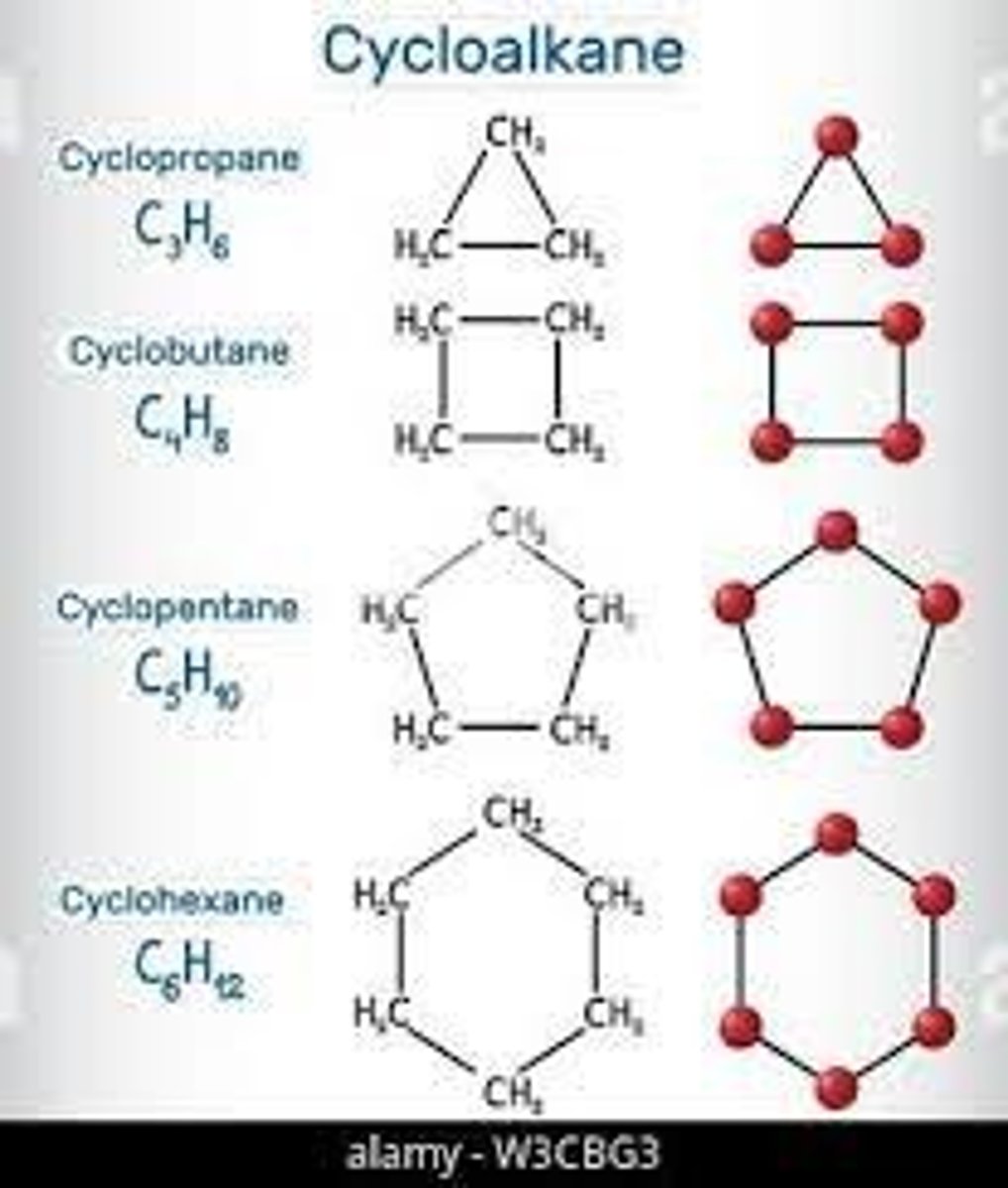
cycloalkanes
are nonplanar with the exception of cyclopropane
cyclopropane is planar and is destabilized by angle and torsional strain
cyclopropane is non planar and is less strained than cyclopropane
cyclobutane
ring puckers to minimise eclipsing strain and is very unstable
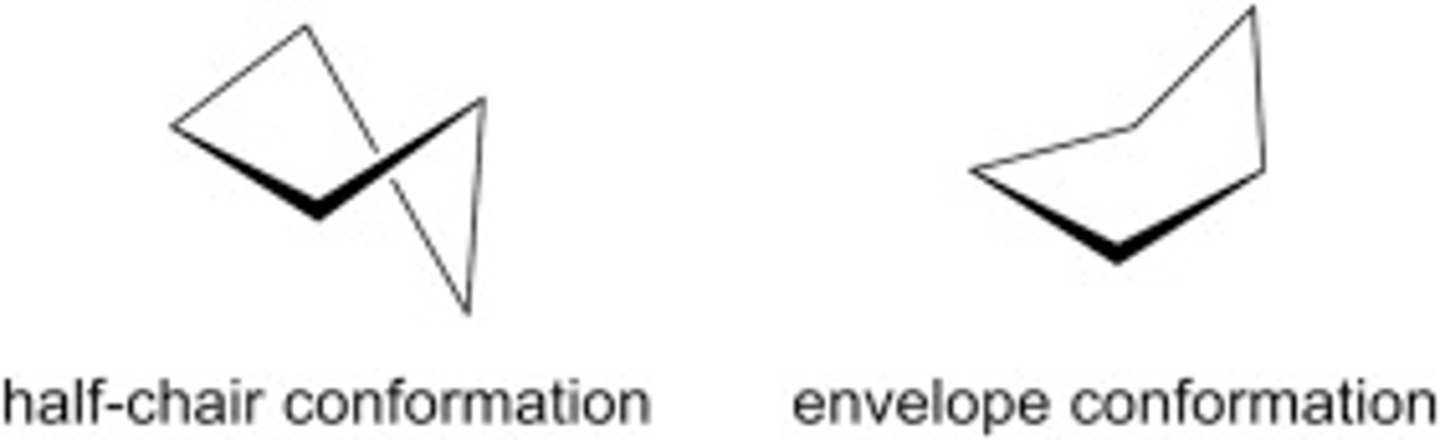
cyclopentane
has 2 non planar conformations that are similar in stability
envelope and Half chair
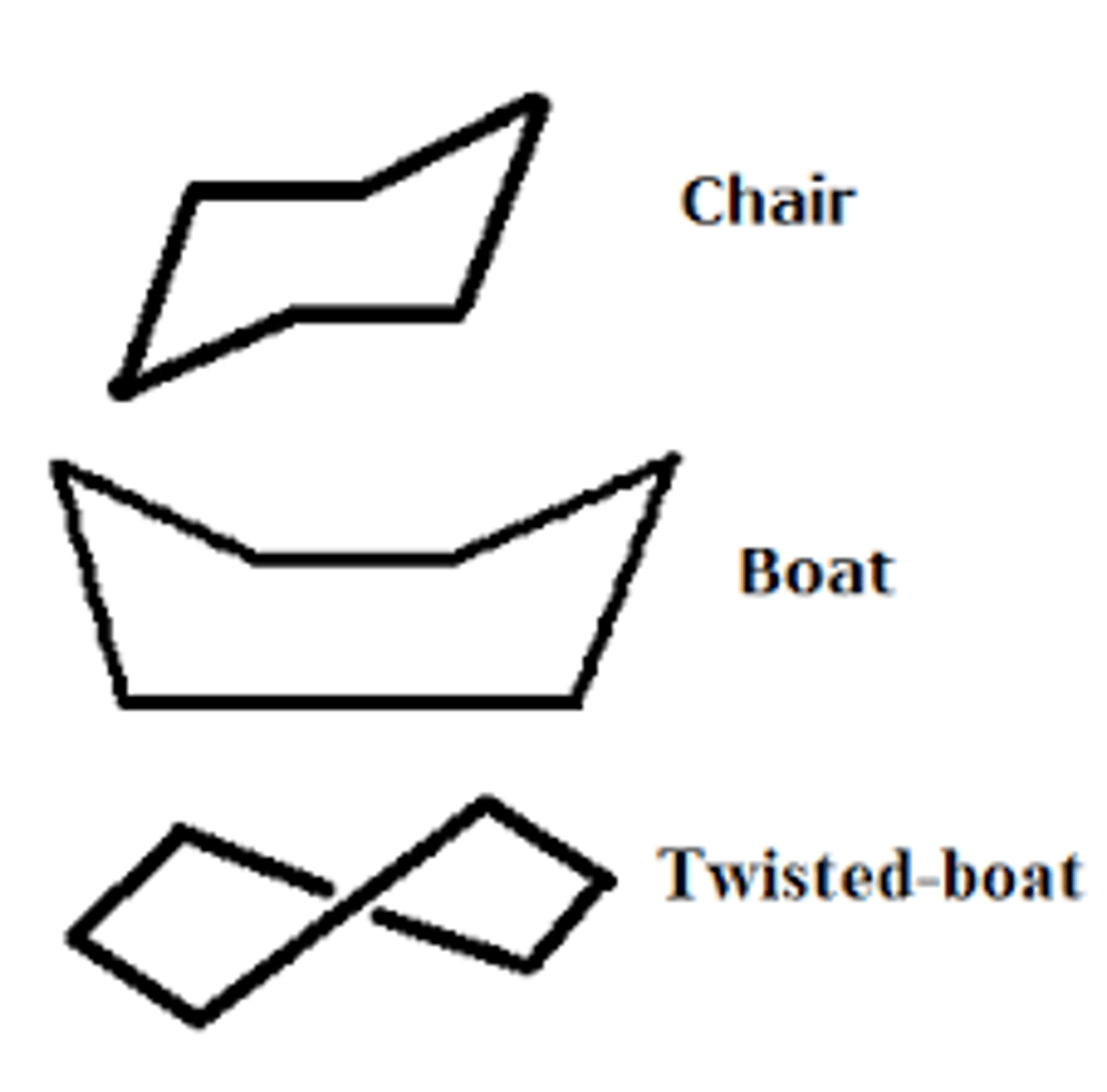
cyclohexane
have tetrahedral angles and there are 3 conformations
chair (most stable no torsional strain), the boat, and the twist
when a cyclohexane ring is present it almost always adopts a chair conformation- most stable
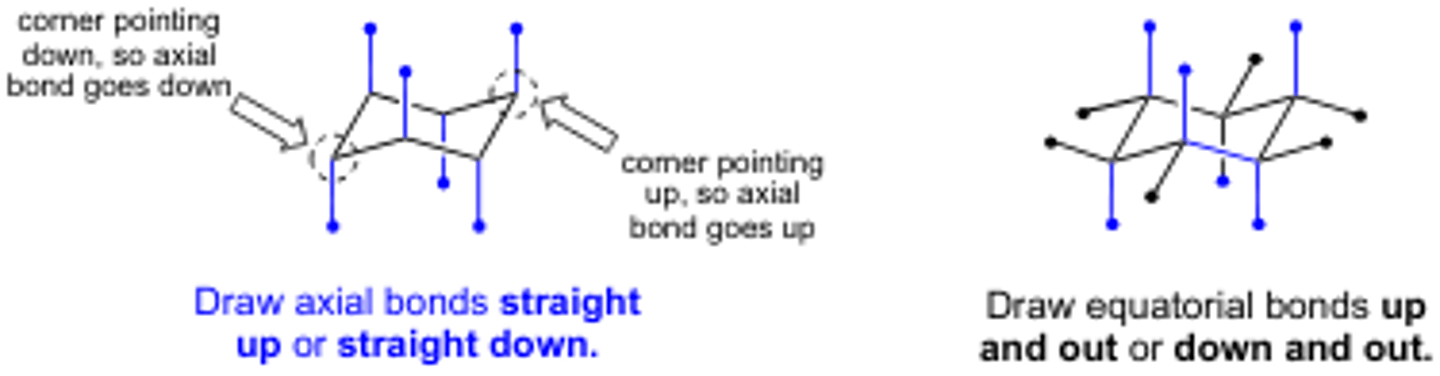
C-H bonds (Axial vs equatorial) in the chair conformation of a cyclohexane
they are not all equivalent and are divided into two sets of 6 each
axial and equatorial
Stereoisomers
Compounds with the same structural formula but with a different arrangement of the atoms in space.
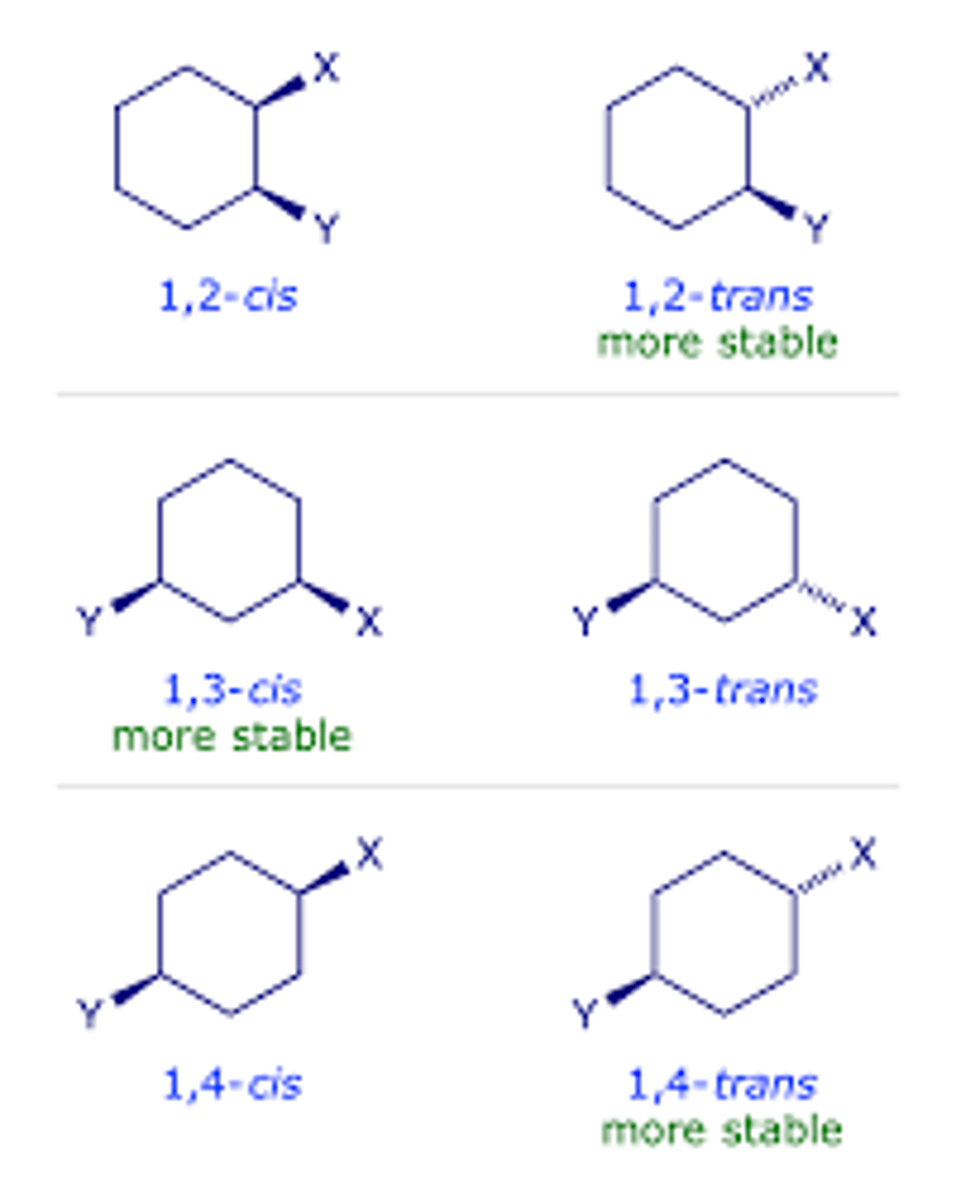
stereoisomer stability
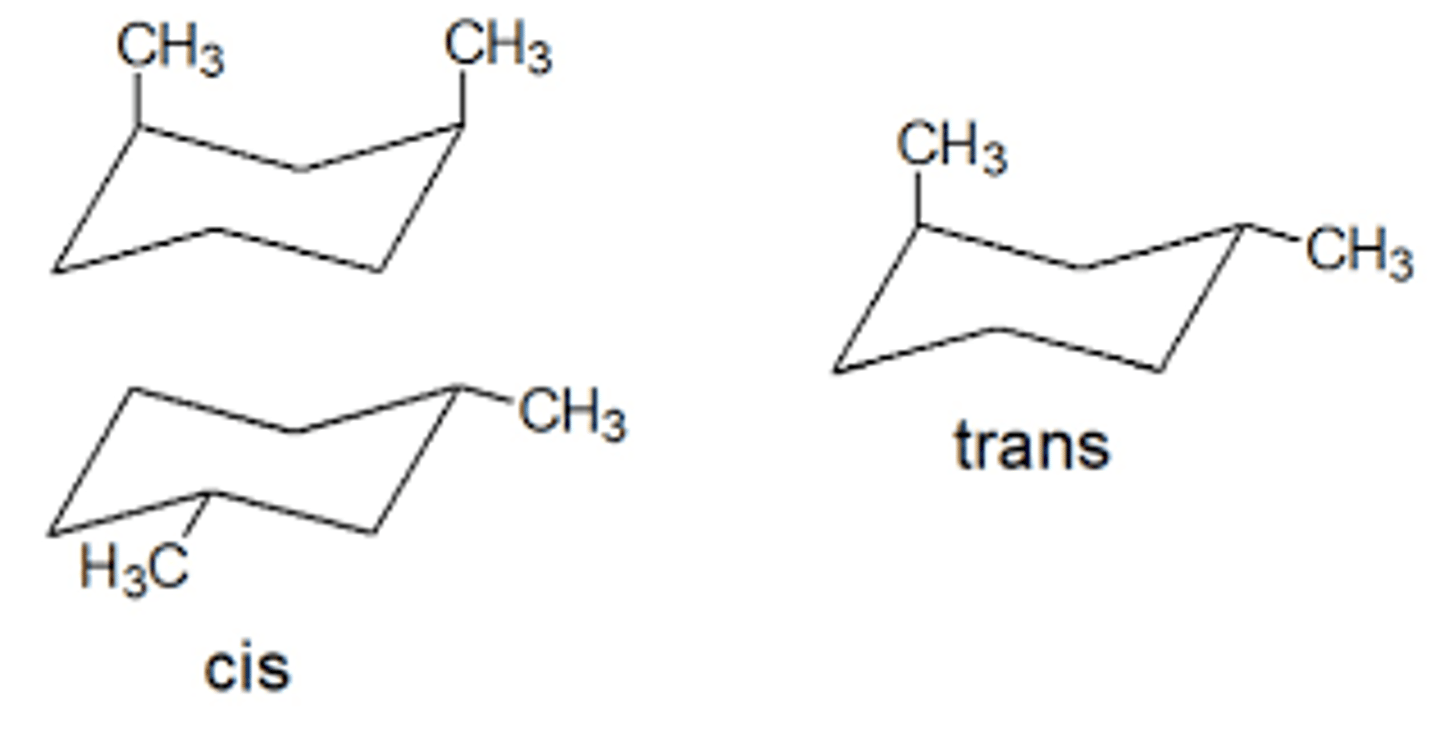
cis-1,3-dimethylcyclohexane vs trans-1,3-dimethylcyclohexane
in this case the trans is more stable because there is one axial methyl group