Isothermal Reactor Design
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
31 Terms
What does isothermal operation imply mathematically?
T = \text{constant} \;\;\Rightarrow\;\; k = \text{constant}
T is temperature
k is the rate constant
Why does reaction rate still change under isothermal conditions?
-rA = f(CA), \quad CA = CA(X_A)
What is the general logic of isothermal reactor design?
\text{Mole balance} + \text{rate law} + CA(XA) \Rightarrow \text{design equation}
What variables are solved for in isothermal reactor design?
Batch: time
CSTR / PFR: volume
What assumptions define an isothermal batch reactor?
F{i,\text{in}} = F{i,\text{out}} = 0
What is the batch reactor design equation in concentration form?
t = \int{C{A,0}}^{CA} \frac{dCA}{-r_A}
t is reaction time
C_{A,0} is initial concentration
What is the batch reactor design equation in conversion form?
t = C{A,0}\int0^{XA} \frac{1}{-rA}\, dX_A
What variables determine batch reaction time?
t = f(-rA, XA)
What key assumption defines an isothermal CSTR?
C{A,\text{reactor}} = C{A,\text{outlet}}
What is the isothermal CSTR design equation?
V = \frac{F{A,0} XA}{-rA|{\text{outlet}}}
V is reactor volume
F_{A,0} is inlet molar flow of A
Why is the CSTR design equation algebraic?
-r_A = \text{constant throughout reactor}
Where is the reaction rate evaluated in a CSTR?
-rA = -rA|_{\text{outlet}}
What key assumption defines an isothermal PFR?
CA = CA(z), \quad \text{no axial mixing}
What is the isothermal PFR design equation?
V = \int0^{XA} \frac{F{A,0}}{-rA}\, dX_A
Why is PFR design an integral equation?
-rA = -rA(X_A)
How does reaction rate vary inside a PFR?
-rA|{XA \approx 0} > -rA|{XA \to X_{A,\text{out}}}
Why does a PFR require less volume than a CSTR for the same conversion?
\langle -rA \rangle{\text{PFR}} > -rA|{\text{CSTR}}
What quantity is plotted in a Levenspiel plot?
\frac{1}{-rA} \;\text{vs}\; XA
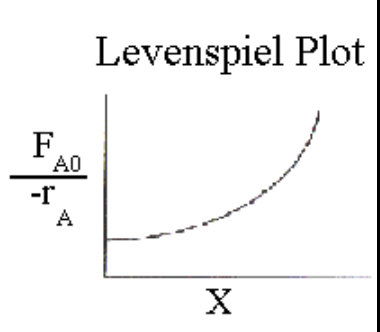
What does the area under a Levenspiel plot represent for a batch reactor?
t = C{A,0}\int0^{XA} \frac{1}{-rA}\, dX_A
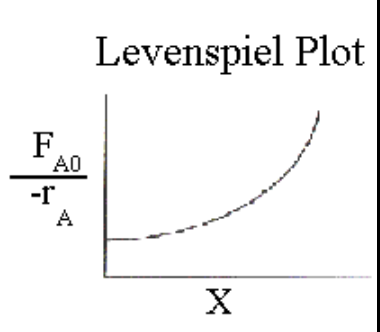
What does the area under a Levenspiel plot represent for a PFR?
V = F{A,0}\int0^{XA} \frac{1}{-rA}\, dX_A
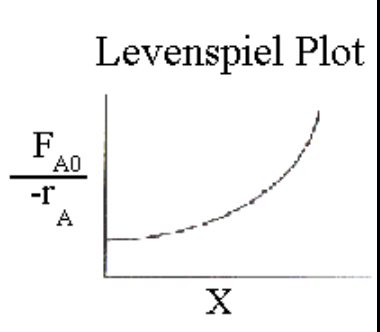
How is a CSTR represented on a Levenspiel plot?
V = F{A,0} XA \left(\frac{1}{-rA}\right){\text{exit}}
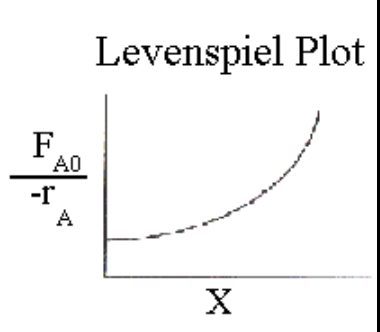
Why do Levenspiel plots show PFR superiority visually?
\text{Area}{\text{PFR}} < \text{Area}{\text{CSTR}}
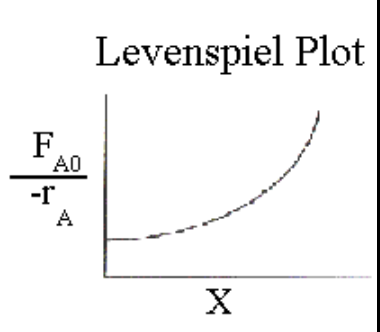
What is the definition of the Damköhler number?
\text{Da} = \frac{\text{reaction rate}}{\text{flow rate}}
What is Da for a first-order reaction?
\text{Da} = k\tau
\tau = \frac{V}{\dot V} is space time
What is the CSTR conversion for a first-order reaction in terms of Da?
X_A = \frac{\text{Da}}{1 + \text{Da}}
What does a small Damköhler number imply?
\text{Da} \ll 1 \Rightarrow X_A \approx 0
What does a large Damköhler number imply?
\text{Da} \gg 1 \Rightarrow X_A \to 1
Why is the Damköhler number useful?
Quick estimation of conversion and reaction vs flow limitation
What effects are excluded from isothermal reactor design?
Temperature change, heat transfer, equilibrium shifts due to temperature
Why is isothermal design considered foundational?
\text{Non-isothermal design} \supset \text{isothermal framework}
One-sentence exam definition of isothermal reactor design
Isothermal reactor design combines mole balances and kinetics at constant temperature to determine the time or reactor volume required to achieve a specified conversion