Chapter 11: Core Organic Chemistry
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
what is an unsaturated hydrocarbon?
contains carbon-carbon multiple bonds
consists of hydrogen and carbon only
what is a saturated hydrocarbon?
has only single carbon-carbon bonds
consists of hydrogen and carbon only
what is a homologous series?
family of compounds with similar chemical properties
successive members differ by CH2
bond angle around a carbon in an alkane?
109.5o
what is a functional group?
part of an organic molecule that is largely responsible for the molecule’s chemical properties
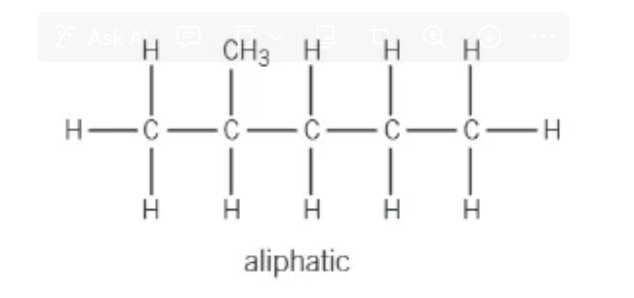
what is an aliphatic hydrocarbon?
straight / branched chains
non-aromatic rings
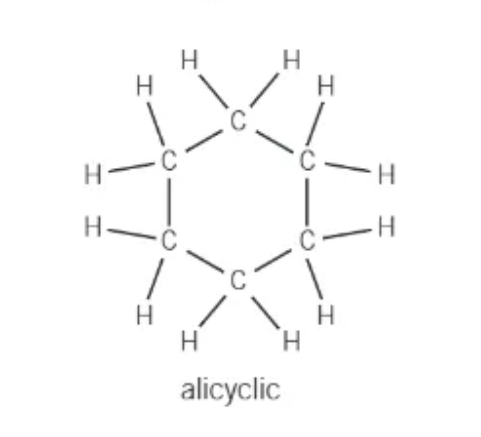
what is an alicyclic hydrocarbon?
joined in a ring → can have branches
what is an aromatic hydrocarbon?
some or all carbons are in a benzene ring
what is an alkyne?
has at least 1 triple bond
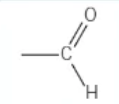
what is this functional group?
aldehyde → [stem]al
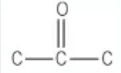
which functional group is this?
ketone → [stem]one
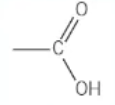
which functional group is this?
carboxylic acid - [stem]oic acid
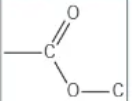
which functional group is this?
ester → [stem]oate
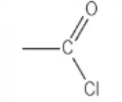
which functional group is this?
acyl chloride → [stem]oyl chloride
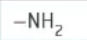
which functional group is this?
amine → [stem]amine OR amino[stem]

which functional group is this?
nitrile → [stem]nitrile
what is the molecular formula?
shows the number and types of atoms of each element present in a molecule
what is an empirical formula?
the simplest whole-number ratio of the atoms of each element present in a compound
what is a general formula?
simplest algebraic formula for any member of a homologous series
what is a displayed formula?
shows the relative position of all the atoms in a molecule + the bonds between them
what is a structural formula?
uses the smallest amount of detail necessary to show the arrangement of atoms in a molecule
what is a skeletal formula?
simplified organic formula:
removes all carbons + hydrogens from carbon chains
removes any bonds to hydrogen atoms
what is a structural isomer?
same molecular formula
different structural formula
if a compound has its functional group attached to a different carbon, is it an isomer?
yes
can aldehydes and ketones be isomers?
yes → if they have the same molecular formula
what is a covalent bond?
a shared pair of electrons between 2 atoms
what is homolytic fission?
each of the bonded atoms takes one of the shared pair of electrons from the bond
each atom now has 1 unpaired electron
forms 2 radicals
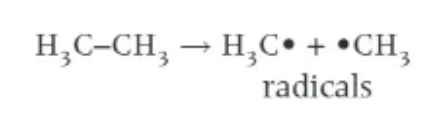
what is a radical?
an atom / group of atoms with an unpaired electron
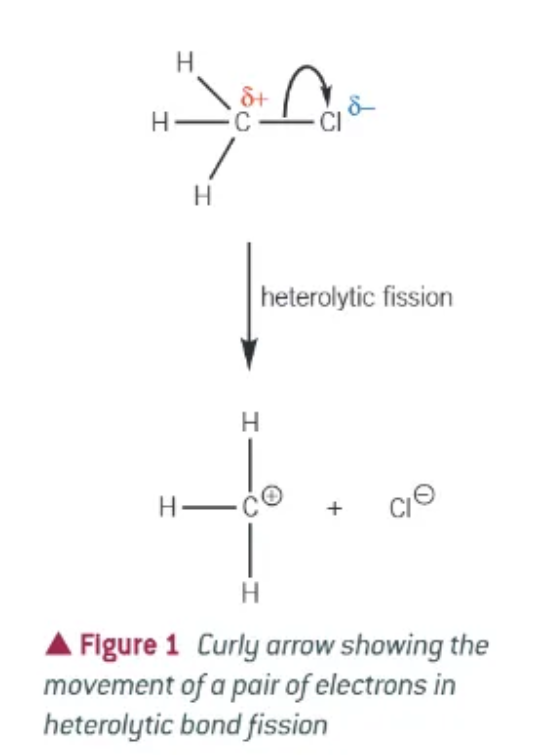
what is heterolytic fission?
one of the bonded atoms takes both of the electrons from the covalent bond
atom that takes both becomes negative ion
atoms that does not becomes a positive ion
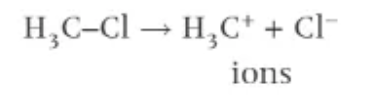
what do curly arrows show?
the movement of a pair of electrons
what happens in an addition reaction?
2 reactants join together to form 1 product
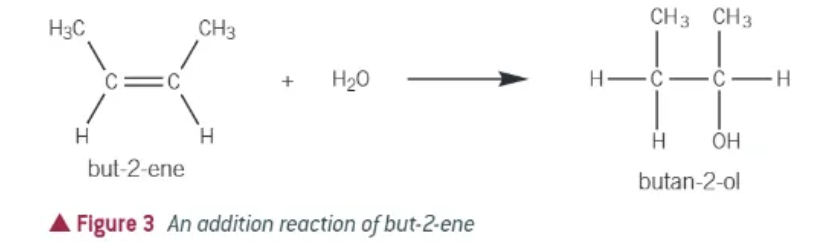
what happens in a substitution reaction?
an atom/group of atoms is replaced by a different atom/group of atoms
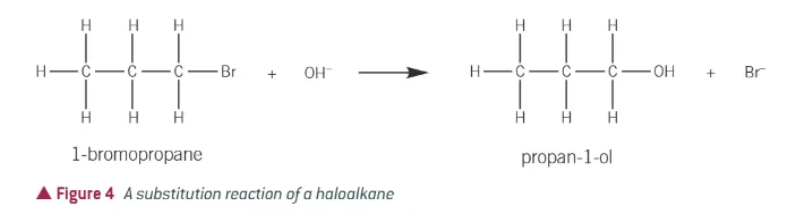
what happens in an elimination reaction?
the removal of a small molecule from a larger one