Lab F: Fischer Esterification
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
45 Terms
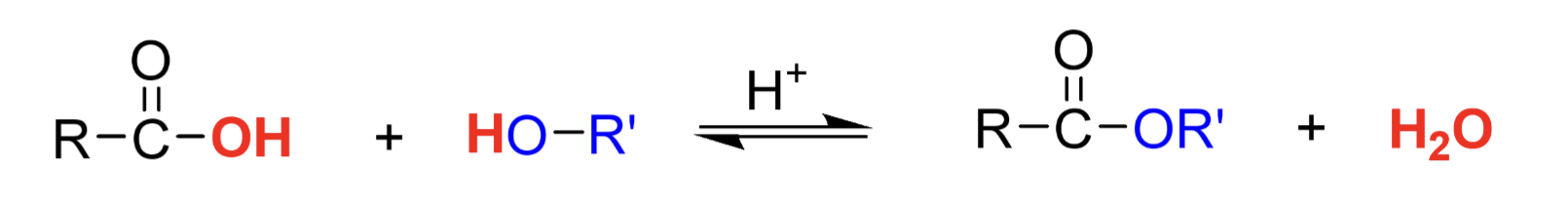
Draw the generic reaction of a Fischer Esterification reaction?
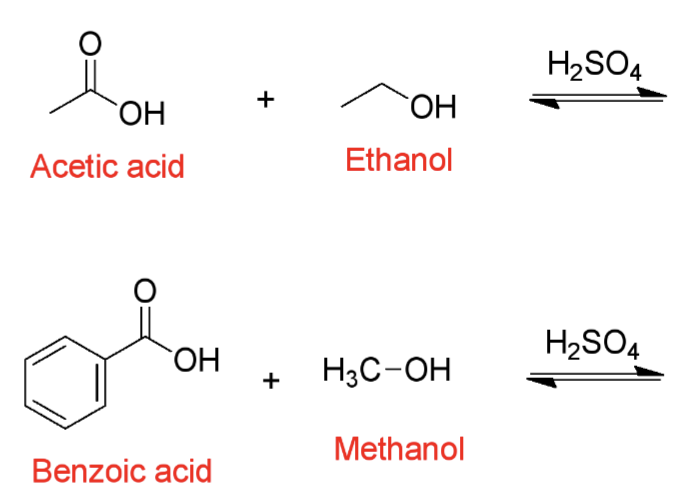
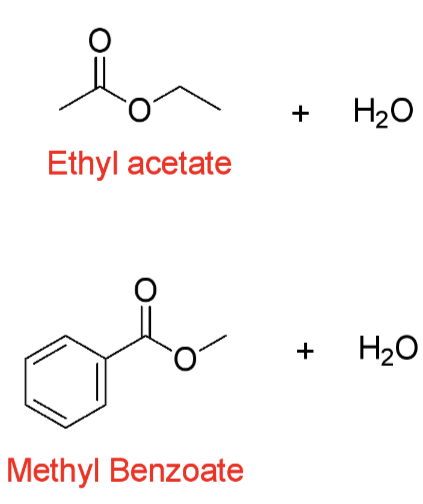
Predict the product of these Fischer Esterification reactions?
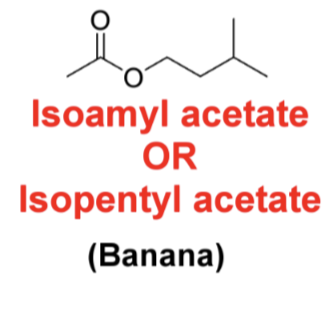
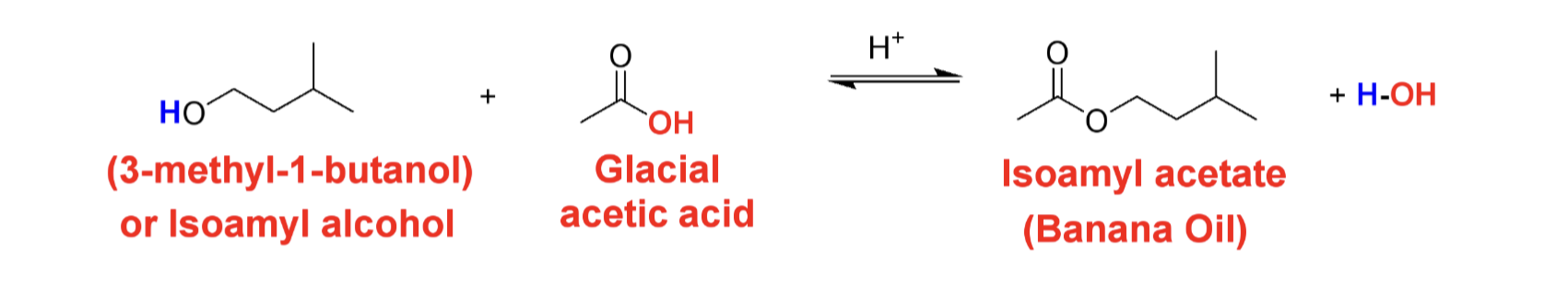
Draw the following structure Isoamyl acetate/Isopentyl acetate (banana)
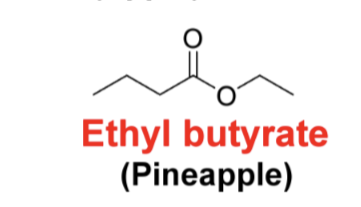
Draw the following structure methyl butyrate (apple)
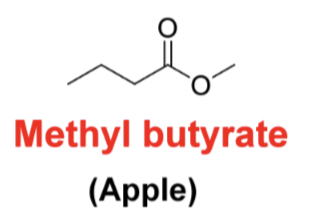
Draw the following structure n-propyl acetate (pear)
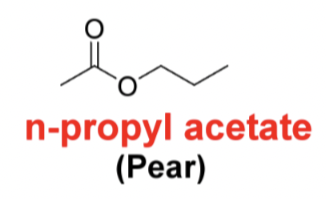
Draw the following structure isobutyl propionate (rum)
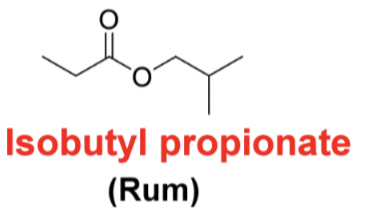
Draw the following structure ethyl butyrate (pineapple)
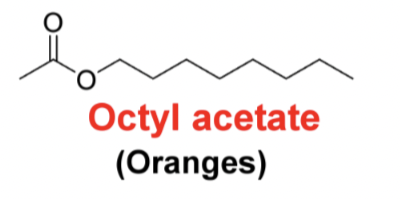
Draw the following structure octyl acetate (oranges)
What are the physical characteristics of esters?
They have pleasant fruity odors, they’re used as flavoring agents in the perfume industry
What are the methods involved to prepare Esters?
Reflux, Extractions, Simple Distillation
What type of reaction is used to prepare esters?
Acid catalyzed reaction which means that the acid is regenerated and is never consumed
Why is it impossible to obtain a 100% yield in preparing esters with an acid catalyzed reaction?
Because the equilibrium state causes the reaction to be reversible which entails that the ester could be protonated again by the trace acid
What do we do in this Fischer Esterification to push the reaction forward?
Use excess reagent which is glacial acetic acid to push the product forward
Draw the reaction of the synthesis of this labs Esterification reaction
Why is glacial acetic acid named with “glacial”?
Because when freezing it forms crystals at room temperature or slightly below room temperature
What is the boiling point of glacial acetic acid?
118 degrees celsius
What are the chemical hazard ratings of glacial acetic acid?
Flammable (rating 2), health (rating 3), corrosive
What is the chemical hazard rating of sulfuric acid?
Corrosive, health (rating 3)
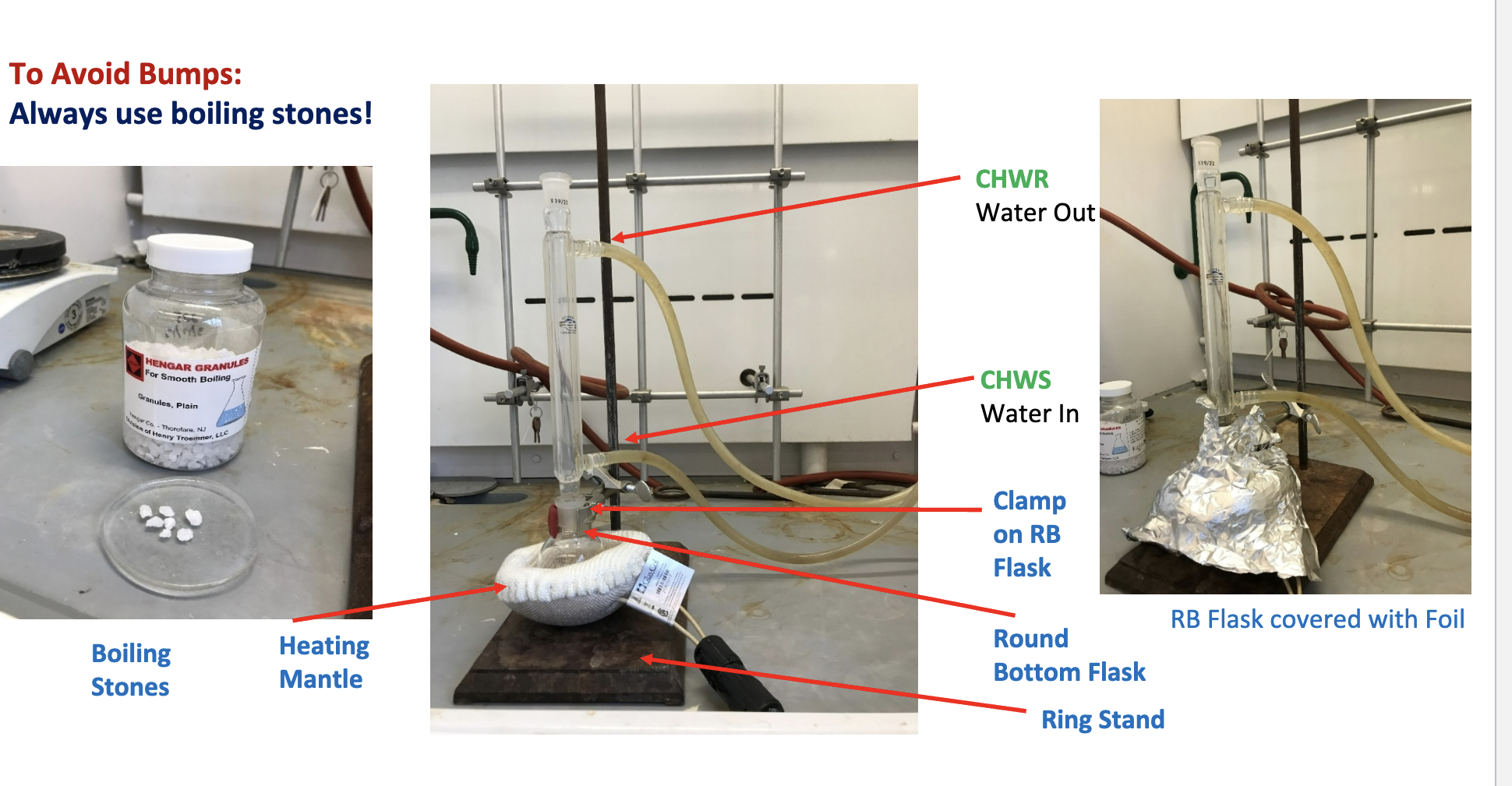
Why do we use boiling stones for the Fischer Esterification reaction during the Reflux?
Because the boiling stones help prevent uneven, choppy, bumping
Why don’t we use boiling stones for the Diels Alder reaction? What do we use instead?
Because crystals will form around the boiling stones which loses product, so we use a stir bar instead.
Draw the reflux reaction for the Fischer Esterification
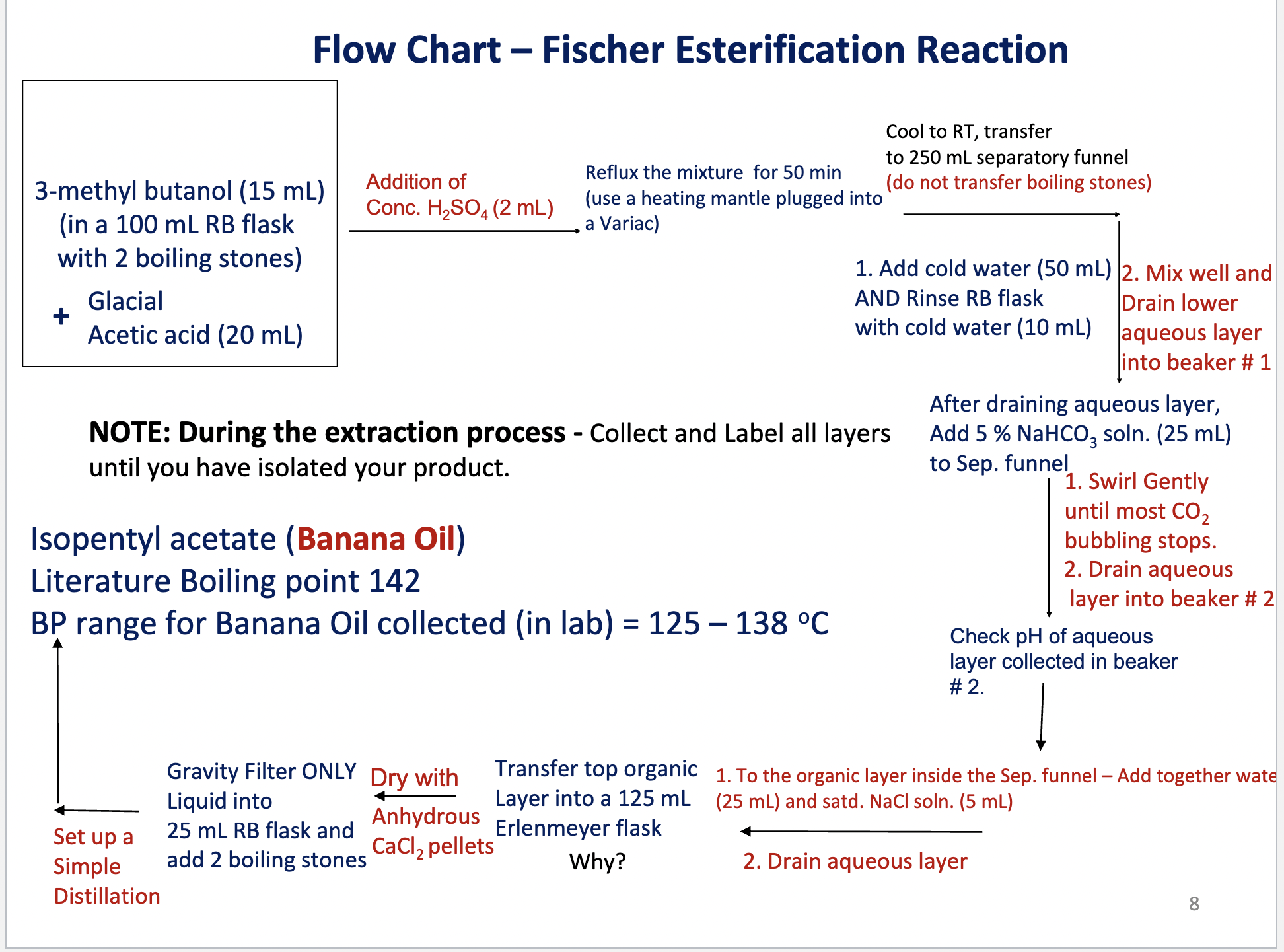
Write the flow chart for the Fischer Esterification reaction
What are the purification techniques?
Recrystallization, Thin Layer Chromatography, Extraction
What is simple distillation used for?
Used to separate liquids where the boiling points differ by greater than 100 degrees celsius at 1 atm, the two liquids must be miscible in each other
What is distillation?
A technique in which a liquid is vaporized by heating to its boiling point, then re-condensed back to a liquid called the distillate which is collected in a receiving flask
Draw the schematic for the Simple Distillation
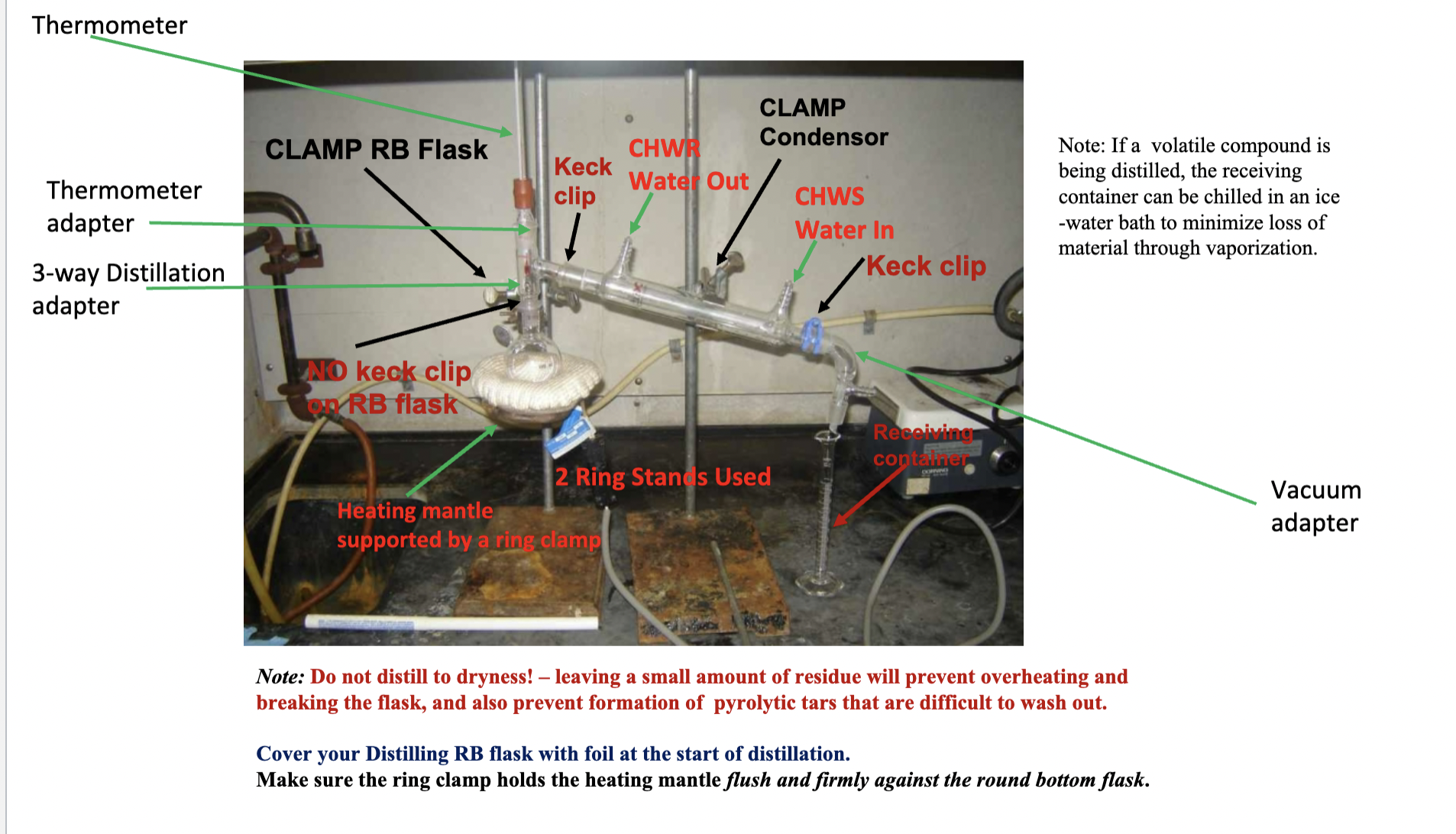
Why should you not distill to dryness?
Because a small amount of residue will prevent overheating and breaking of the flask, prevent pyrolytic tars that are hard to wash out
What are some examples of what the distillation technique can be used for from decreasing to increasing in density and boiling point?
petroleum gas, chemicals, petrol for vehicles, jet fuel, diesel fuels, lubricating oils, fuels for ships, bitumen for roads
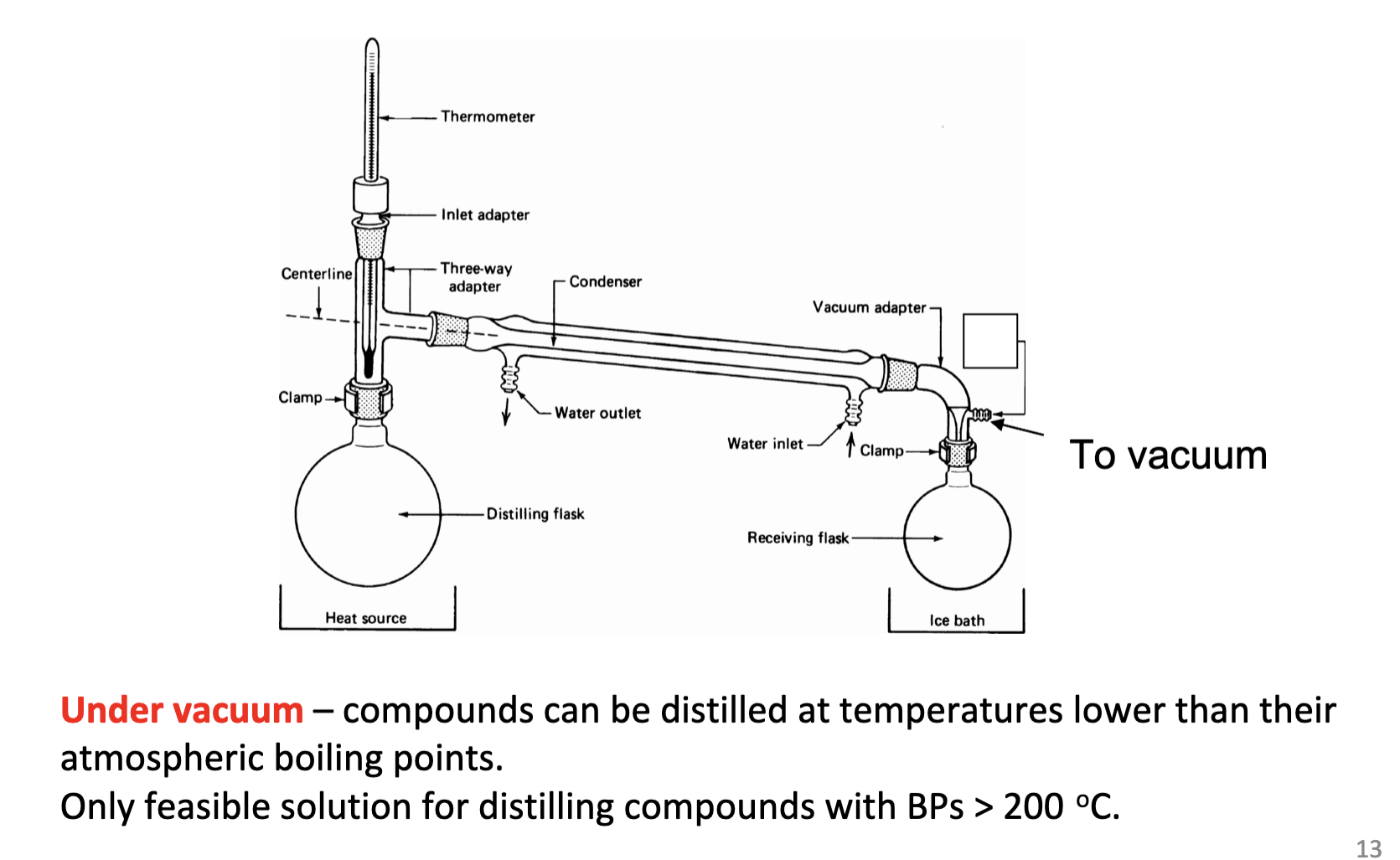
What is vacuum distillation used for?
Its used for compounds that boil at too high a temperature or decompose near their boiling points.
When can compounds be distilled at temperatures lower than their boiling points for vacuum distillation?
When its under the vacuum
What boiling point can a vacuum distillation be distilled at?
Greater than 200 degrees celsius
Draw the schematic for a vacuum distillation
What is a fractional distillation used for?
Its used to separate liquid mixtures where the difference in boiling points is less than 100 degrees celsius at 1 atm
What is unique about the fractional distillation?
It uses a Vigreux column which has indentations that allow several simple distillation cycles to occur, so many vaporizations and condensations happen before the distillate is collected.
What is bad and good about fractional distillation?
Its bad for yield but good for efficiency through theoretical plates.
What are the factors affecting Boiling Points?
Size (molecular weight) and intermolecular interactions
List the following structures in order from low to high boiling points (hexane, heptane, pentane)
pentane, hexane, heptane
List the following structures in order from low to high boiling points (butanol, pentane, butyraldehyde)
pentane, butyraldehyde, butanol
What does it mean for a solution to boil?
This is defined as the temperature at which a liquids vapor pressure equals 1 atm, where the vapor pressure equals the atmospheric pressure.
What is the Clausius-Clapeyron Equation
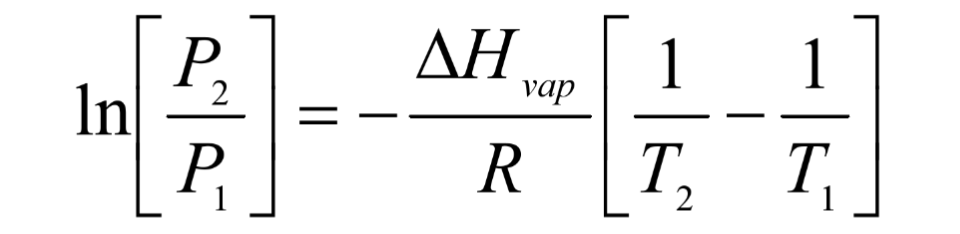
What must you ensure when using a keck clip?
You must make sure the joints are tight because loose joints will cause your product to escape.
Where should the thermometer be placed?
The bulb should be placed below the tangent line (19/22) or below the 3 point distillation adapter space.
What is a Variac?
A Variac is a variable voltage transformed which provides a voltage adjusted source of alternating current.
Where should the Variac be plugged in at?
The Variac outlets below the fume hood not the wall outlets.
What is the rule regarding voltage for a Variac?
The higher the boiling point, the greater the voltage required where the voltage can start at 65.