OCHEM 237 Midterm II | E/Z, R/S, IUPAC
1/20
Earn XP
Description and Tags
Oh man
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
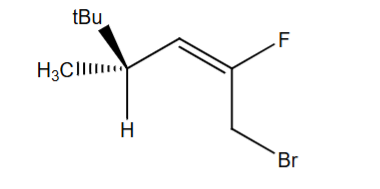
Assign each chiral center in the molecule as R or S, and each alkene as E or Z. You must show work to earn credit.
1 chiral center - S
E notation
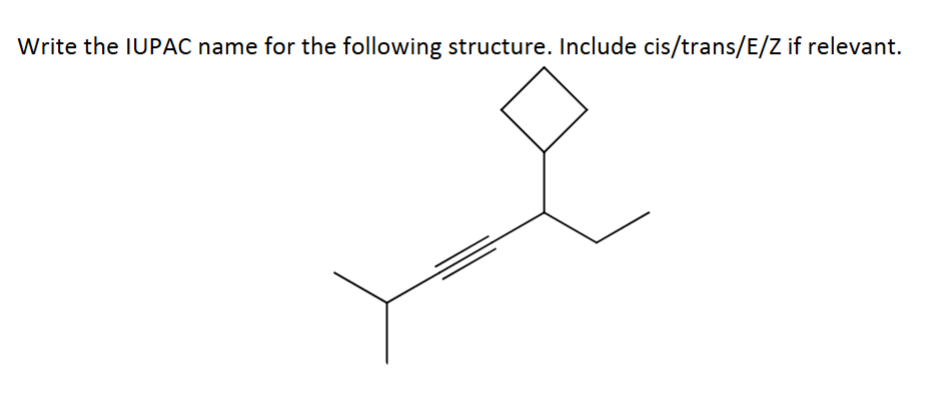
Name it
5-cyclobutyl-2-methylhept-3-yne
Why is the cis/trans nomenclature system not relevant for alkynes?
Alkynes are linear so the 2 groups must be directly opposite each other
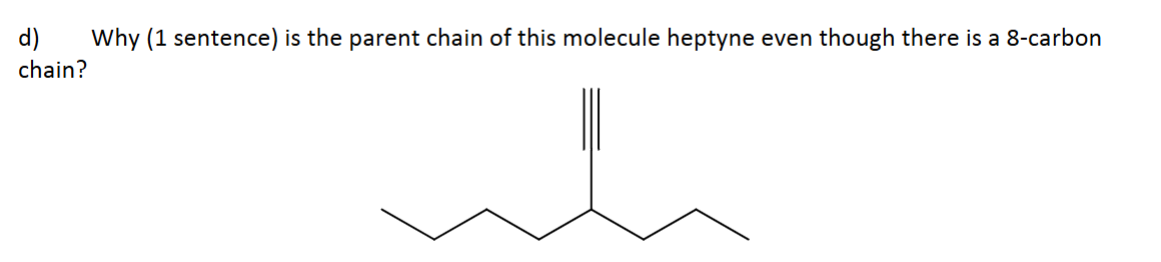
Why?
The alkyne is the most important functional group, so it must be in the parent chain regardless of length.
Draw 3-pentylcyclobutene. Keep track of E/Z/cis/trans
i.) Why is a number not needed for the alkene?
ii) Why is E/Z not needed for the alkene?
iii) The substituent is larger than the ring, why is the parent not pentane?
i) As the most important functional group inside the ring, by definition the alkene will be between C1 and C2.
ii) It’s not geometrically possible to draw an E (trans) cyclobutene. Since 1 geometric isomer is impossible, there’s no ambiguity and no need for E/Z
iii) Alkenes have higher priority than alkanes, so the parent is the largest group that contains the alkene.
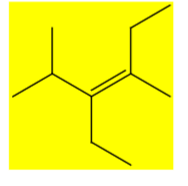
Draw (Z) 3-ethyl-2,4-dimethylhex-3-ene. Keep track of E/Z/cis/trans
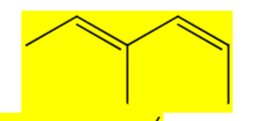
Draw (2E, 4Z) 3-methylhexa-2,4-diene. Keep track of E/Z/cis/trans
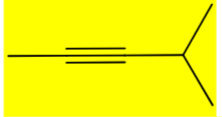
Draw 4-methylpent-2-yne. Keep track of E/Z/cis/trans
i) Why is E/Z/cis/trans not needed for alkynes?
ii) Why not 2-methylpent-3-yne which has lower overall numbering?
i.) Alkynes are linear so there is no same/opposite orientation of groups to worry about
ii.) The pi bond takes top priority for determining the numbering, regardless of other substituent
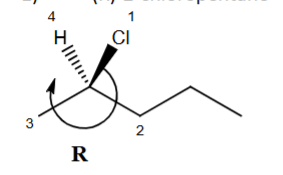
Draw (R)-2-chloropentane using dash-wedge notation
Draw (S)-bromochlorofluoroiodomethane using dash-wedge notation

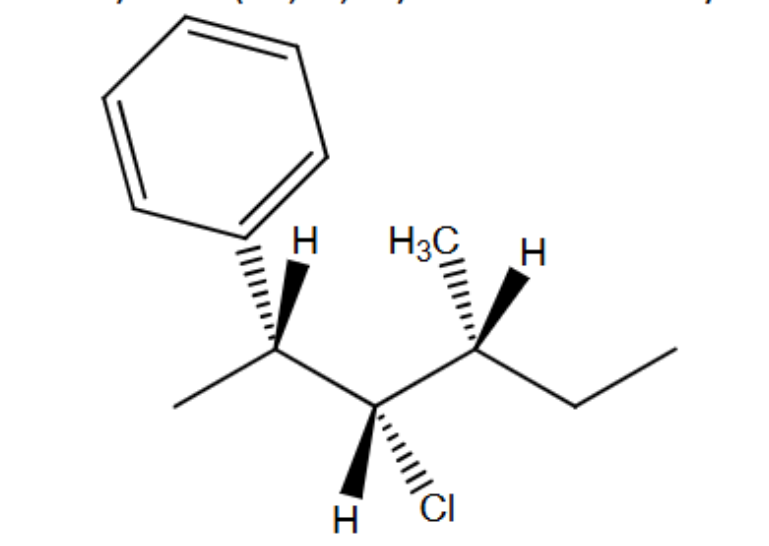
Draw (2S,3R,4S) 3-chloro-4-methyl-2-phenylhexane using dash-wedge notation

Draw (2R,3S,4R) 3-chloro-4-methyl-2-phenylhexane using dash-wedge notation
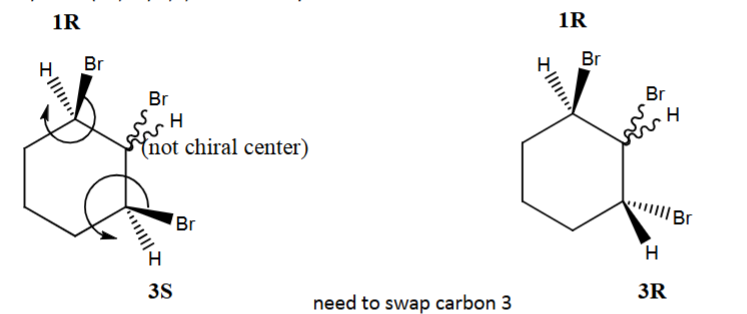
Draw (1R,3R) 1,2,3-tribromocyclohexane using dash-wedge notation
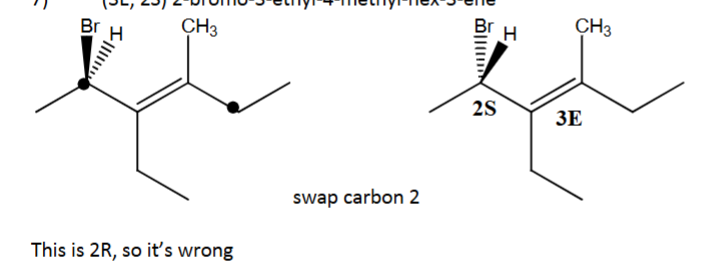
Draw (3E, 2S) 2-bromo-3-ethyl-4-methyl-hex-3-ene using dash-wedge notation
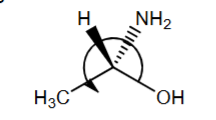
R or S?
R
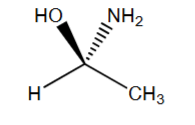
R or S?
R
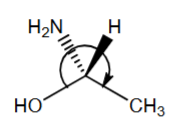
R or S?
S
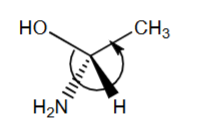
R or S?
R
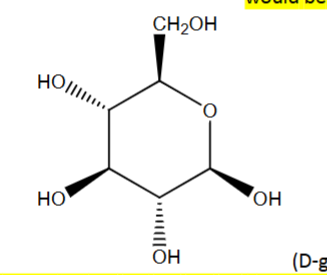
R or S?
1R, 2R, 3S, 4S, 5R
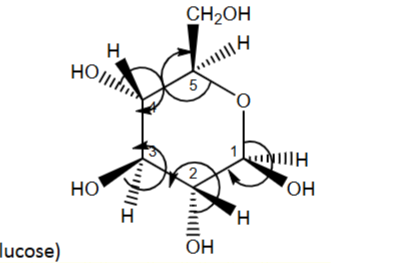
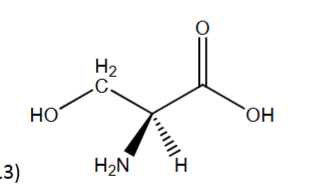
R or S?
S
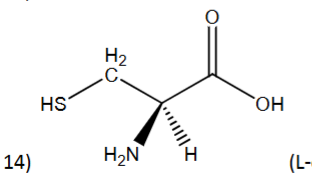
R or S?
R