Entropy and Gibbs Energy
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
what is entropy ?
A measurement of disorder or randomness, the more disordered something is , the more enthalpy it has
what is the units for entropy ?
JK-1 / mol -1
what is the order of entropy from solid → gas
Solid → least entropy
Liquid
Gas → most entropy
as temperature increases, does the entropy increase or decrease ?
Entropy increases
what is the 3rd law of thermodynamics?
at absolute zero, -273*c or 100 kelvin , a crystal has perfect order so zero entropy.
Dissolution- what happens to the entropy ?
Dissolving a solid also increases its entropy — dissolved particles can move freely as they're no longer held in one place:
Number of particles- what happen to the entropy if you increase the number of particles ?
More particles means more entropy. It makes sense — the more particles yeaven i the ore way to and the energy an a seanged. So in a moles
calculating the entropy change - what is the formula ?
🔺S0 = S products - S reactant
Units JK-1 / mol -1
example: 4AI + 3CO2 → 2AI2 O3
🔺S = 2 (51) - [ 4(28) - 2(51)
= - 625 JK-1 / mol -1
what makes a reaction feasible ?
a equation is feasible when it can occur at a particular temperature.
what makes a reaction spontaneous ?
a reaction is spontaneous when it’s feasible and it cane occur under standard conditions.
100 KPa and 298 K.
what is the Gibbs free energy? know as the free energy change
Free-energy change, 🔺G, is a measure used to predict whether a reaction is feasible.
what does the Gibbs free energy take into account?
Free-energy change takes into account the changes in enthalpy and entropy in the system.
what is the formula for free energy change ?
🔺G = 🔺H - T🔺S system
🔺G is negative is it feasible or not
for a reaction to be feasible the🔺 G must be negative therefore at 🔺G = 0 → reaction feasible.
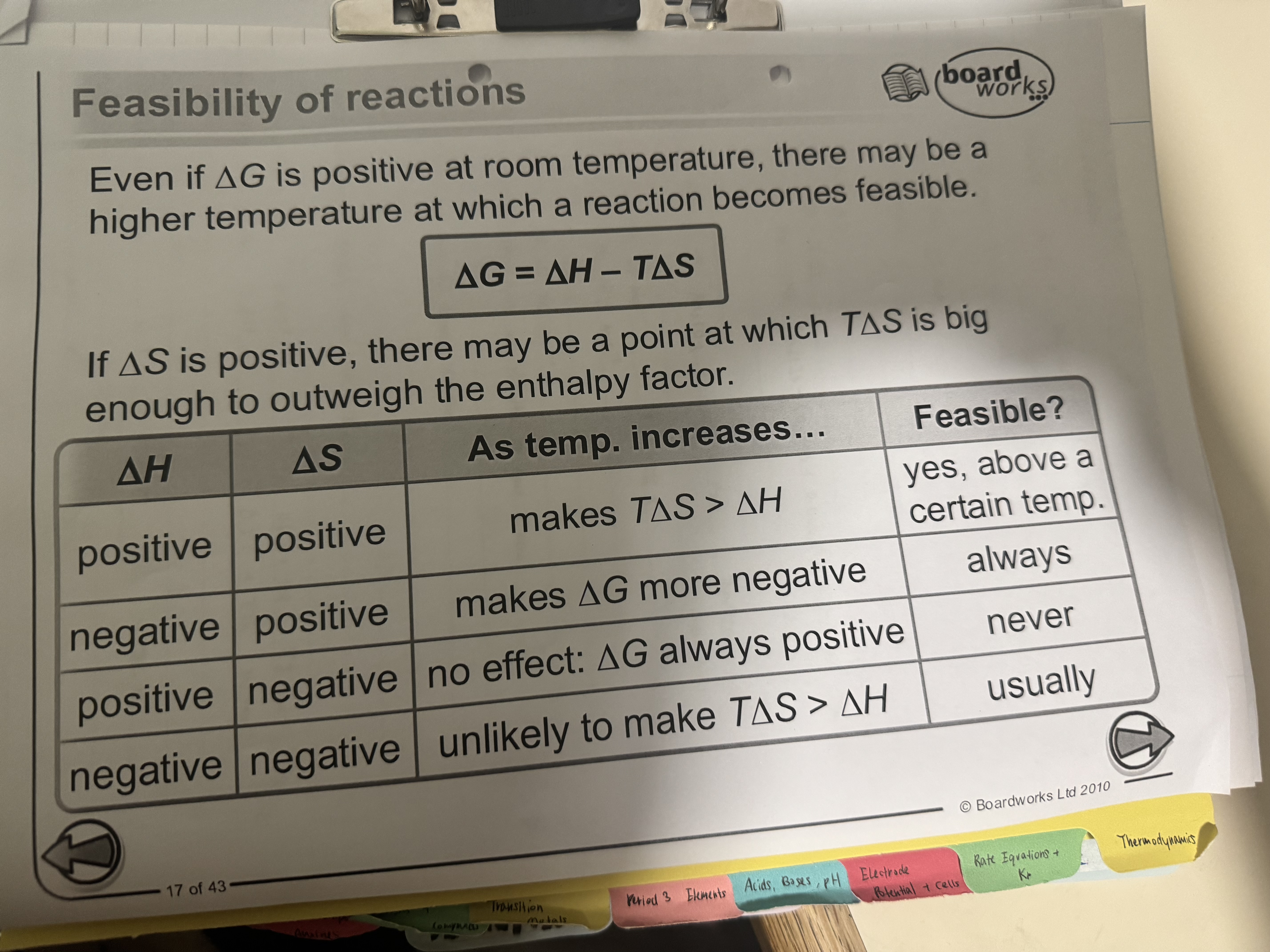

effect on temperature on entropy
entropy contribution of the overall energy depends on temperature
as temp increases, what happens to the entropy change ?
As temp increases, the energy from the entropy components of a reaction becomes more significant.
🔺H & 🔺S are both negative
most exothermic reactions are spontaneous even if entropy decreases. This is because enthalpy contributes more to 🔺G than enthalpy does.
Exceptions are reactions at very high temperature where entropy decreases.
convert 🔺H KJ mol -1 ?
x 1000
convert 🔺S KJ mol -1 ?
divide by 1000 to kj mol -1
thermodynamics vs kinetics
🔺G = negative value → feasible but piece of carbon doesn’t spontaneously burn if left on the table. Energy would need to be put in for the reaction to begin. A reaction that’s thermodynamically feasible isn’t necessary kinetically feasible.
if 🔺H is + and 🔺S + at what temp would the reaction be feasible ?
if 🔺H is + and 🔺S + then the reaction will only be feasible above certain temp.
if 🔺H is - and 🔺S - at what temp would the reaction be feasible ?
if 🔺H is - and 🔺S - then the reaction will only be feasible below certain temp
free energy graph
🔺G = 🔺H - T🔺S
🔺G = ( -🔺S x T) + 🔺H
y = mx + c
