5.3, 5.4- Acids, Bases and pH, buffers and neutralisation
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
bronsted-lowry theory
acid is a substance that donates protons (H+)
base is substance that accepts protons (H+)
how are acids categorised
based on their capacity to donate protons:
monoprotic→ donates 1 proton per molecule
diprotic→ donates 2 protons per molecule
triprotic→ donates 3 protons per molecule
conjugate acid-base pairs
consists of 2 species that are interconverted by transfer of H+
in forward reaction, acid donates proton, but in reverse reaction conjugate base accepts proton
in forward reaction, base accepts proton, but in reverse reaction conjugate acid donates proton

water
water is amphiprotic→ can behave as both acid or base
when reacting with acids, water accepts proton to form H3O+ (hydronium ion)
when reacting with bases, water donates proton to form OH- (hydroxide ion)
metal and acid
salt and hydrogen
redox reaction
metal carbonate and acid
salt, water and CO2
neutralisation
insoluble bases and acid
salt and water
neutralisation
acids and alkalis
salt and water
neutralisation
development acid-base theories
lavoisier:
proposed that all acids contain oxygen
doesn’t apply to acids e.g. HCl
Arrhenius:
acids release H+ in water and bases release OH-
does not account for bases e.g. NH3 that do not release OH-
the pH scale
logarithmic scale that measures concentration of H+ in a solution
ranges from 0-14:
<7 is acidic solution
=7 is neutral
>7 is alkaline solution
calculating pH (+ inverse)
pH=-log10[H+]
[H+]=10-pH
H+ concentration in different strong acids
Monoprotic→ [H+]=[acid]
Diprotic→ [H+]= 2[acid]
dissociation of H+ in weak acids
partially dissociate in aqueous solution
conc of H+ less than initial conc. of acid
acid dissociation constant (Ka)
Ka= [H+][A-]/[HA]
larger Ka= stronger the weak acid
assumptions made for weak acids
[HA]equilibrium≈[HA]initial → ionisation of weak acid is so small that conc. of undissociated HA at equilibrium is approx. same as initial conc.
[H+]equilibrium≈[A-]equilibrium → ionisation of water is negligible
assumptions simplify Ka formula to Ka=[H+]2/[HA]
when do Ka assumptions become inaccurate
for stronger acids withe Ka>10-2, conc H+ is significant so [HA]equilibrium≠[HA]initial
for very weak acids or very dilute solutions, conc H+ from dissociation of water becomes significant so [H+]equilibrium≠[A-]equilibrium
pKa
pKa=-log10(Ka)
what is a buffer solution
a solution that minimises change in pH on addition of small amounts of acid or base.
type 1 buffers
weak acid (HA)
salt of the weak acid/ conjugate base of weak acid (A-M+)
type 2 buffers
weak acid in excess (xs HA)
small amount of strong alkali (M+OH-)
how do buffers minimise pH change on addition of acid
more acid= increased [H+]
based on Le Chatelier’s principle, equilibrium position shifts to left
more H+ ions react with CH3COO- to form CH3COOH
causes [H+] to decrease
how do buffers minimise pH change on addition of alkali
when alkali added, increase in [OH-]
OH- react with H+ to form water
causes [H+] to decrease
equilibrium will shift to the right
more CH3COOH dissociate, so [H+] increases
calculating pH of type 1 buffer solutions
cannot simplify to [H+]2 because [A-]>[H+]
We can assume [HA](equilibrium)= [HA]original
![<ul><li><p>cannot simplify to [H<sup>+</sup>]<sup>2</sup> because [A<sup>-</sup>]>[H<sup>+</sup>]</p></li><li><p>We can assume [HA]<sub>(equilibrium)</sub>= [HA]<sub>original</sub></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/23c66046-edd2-40d4-af64-eee4009a3b6a.png)
calculating pH of type 2 buffers
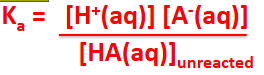
what range does blood plasma need to have a pH between
7.35-7.45
below= acidosis
above= alkalosis
components of carbonic acid-hydrogen carbonate buffer system
carbonic acid (H2CO3)- weak acid
Hydrogen carbonate ion (HCO3-)- conjugate base
blood buffer equation
H2CO3(aq) ⇌ H+(aq) + HCO3-(aq)
what happens if there is an increase in H+ in blood buffer system
[H+] increases
extra H+ reacts with HCO3- to form H2CO3
position of equilibrium shifts to left to remove most of the extra H+
what happens when there is an increase of OH- in blood buffer system
increases [OH-]
extra [OH-] reacts with H+ to form H2O
decreases H+
equilibrium position shifts to the right to restore most of used up H+
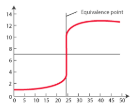
understanding titration curves
starts at pH below 7→ shows that acid in conical and alkali in burette
ends at pH above 7→ shows there is an excess of alkali at end of titration
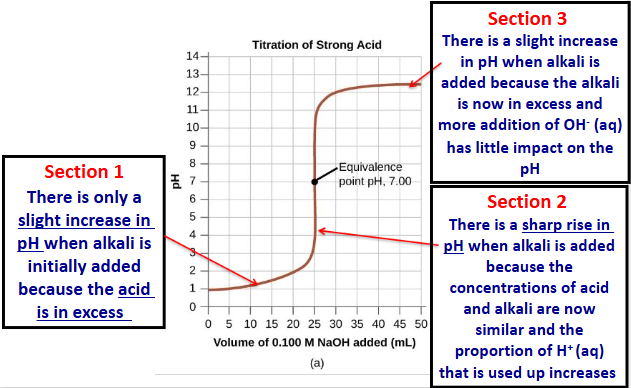
sections of a titration curve
only slight increase in pH when alkali is added because acid is in excess
sharp rise in pH when alkali is added because concentrations of acid and alkali are now similar and proportion of H+ used up increases
slight increase in pH when alkali added because alkali is in excess and more addition of OH- has little impact on pH
equivalence point
the volume of solution that completely reacts (and therefore neutralises) the volume of the other solution
finding equivalence point on titration curve
find centre of vertical section
draw a vertical line going down x-axis to identify corresponding volume
strong acid strong base titration curve
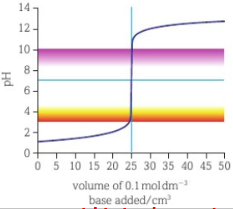
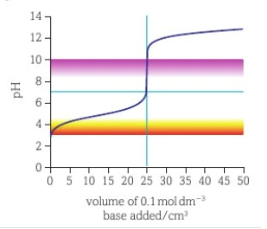
strong acid weak base titration curve
end of curve shifted down- weak base has lower pH
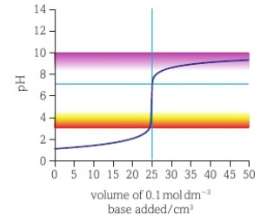
weak acid strong base titration curve
start of graph shifted up- weak acid has higher pH
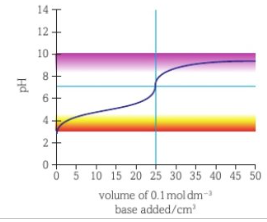
weak acid weak base titration curve
start shifted up, end shifted down
what are pH indicators
weak acids in solution
conjugate weak acid has a different colour to its conjugate base
what is end point of a titration
equal concentrations of weak acid and conjugate base forms of the indicator→ colour chnage observed
choosing suitable indicators
indicator must have an end point colour change that coincides with equivalence point