Entropy and enthalpy
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
24 Terms
Lattice enthalpy definition
The enthalpy change that accompanies the formation of 1 mol of an ionic lattice from its gaseous ions under standard conditions
Is lattice enthalpy exo or endo and why
Lattice enthalpy is exothermic because strong electrostatic forces are formed between the oppositely charged ions to achieve a more stable state
Standard enthalpy change of formation definition
The enthalpy change that accompanies the formation of 1 mol of a substance from its constituent elements in their standard states under standard conditions
What is standard enthalpy of atomisation
The enthalpy change when changing an element into a monoatomic gas
Is enthalpy change of atomisation endo or exo
Endothermic because energy is required to break bonds to form a gas
First ionisation energy definition
The minimum energy required to remove 1 electron from each atom is 1 mol of gaseous atoms to form 1 mol of gaseous 1+ ions
Is ionisation endo or exo
Endothermic because energy is required to overcome the electrostatic forces between the positive nucleus and the negative electron
What is an electron affinity
When an electron is added to an atom/ion
Is the first electron affinity exo or endo
First electron affinity is exothermic as the negative electron is attracted to a positive nucleus
Is second electron affinity endo or exo
Endothermic because energy is required to force an electron toward a negatively charged ion
Standard enthalpy change of solution definition
The enthalpy change accompanying 1 mole of a solute being completely dissolved in a solute Iunder standard conditions
Is enthalpy change of solution endo or exo
Can be either depending on the relative magnitudes of the enthalpy change of hydration and lattice enthalpy
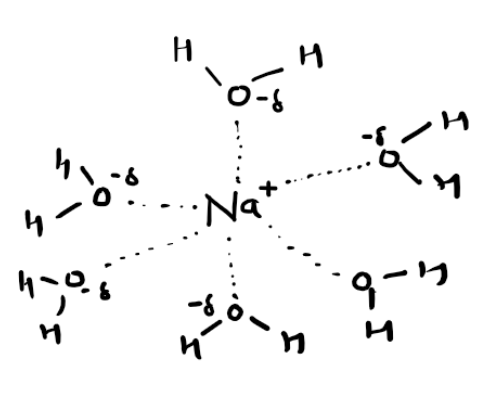
How to draw a dissolved ion
How to find enthalpy change of solution experimentally
Put a thermometer in a polystyrene cup with water and add the ionic solid, use the mass of the solution and c of water in q=mcT to find enthalpy
Enthalpy change of hydration definition
The enthalpy change that accompanies 1 mole of gaseous ions dissolving in water to form 1 mol of aqueous ions under standard condition
What properties of an ion form a stronger lattice
Higher charge, smaller atom (higher charge density)
Ionic size trends across a period
Cations decrease in size left to right, anions decrease in size across the period
What properties of ions cause them have more exothermic hydration enthalpies
smaller ionic size, larger ionic charge (as stronger bonds are formed)
What is entropy
The amount of disorder that there is in a system, a measurement of how the energy is spread outW
Standard entropy definition
Entropy of a substance under standard condition
Entropy of a substance at absolute zero
0, so all other entropys must be positive
What is the entropy change in the changing of states
Entropy change is positive when a substance becomes more random, so solid → liquid or liquid→gas
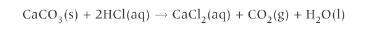
Would entropy change be +ve or -ve
+ve because a gas has formed which has more entropy

Would entropy change be +ve or -ve
-ve because there are less gaseous moles formed than started