WJEC AS Chemistry Unit 1.2 - Basic Ideas About Atoms
1/74
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
75 Terms
Atomic number
number of protons in the nucleus
number of protons=number of electrons
Mass number
number of protons and neutrons in the nucleus
Isotopes
atoms with the same number of protons but different numbers of neutrons
Types of radioactive emission
Alpha
Beta
Gamma
Alpha particles
Have a nucleus of 2 protons and 2 neutrons, therefore positively charged.
Alpha emissions
least penetrating of the three types
Stopped by a thin sheet of paper
Strongly ionising because they are large, relatively slow and carry two positive charges
Alpha symbol
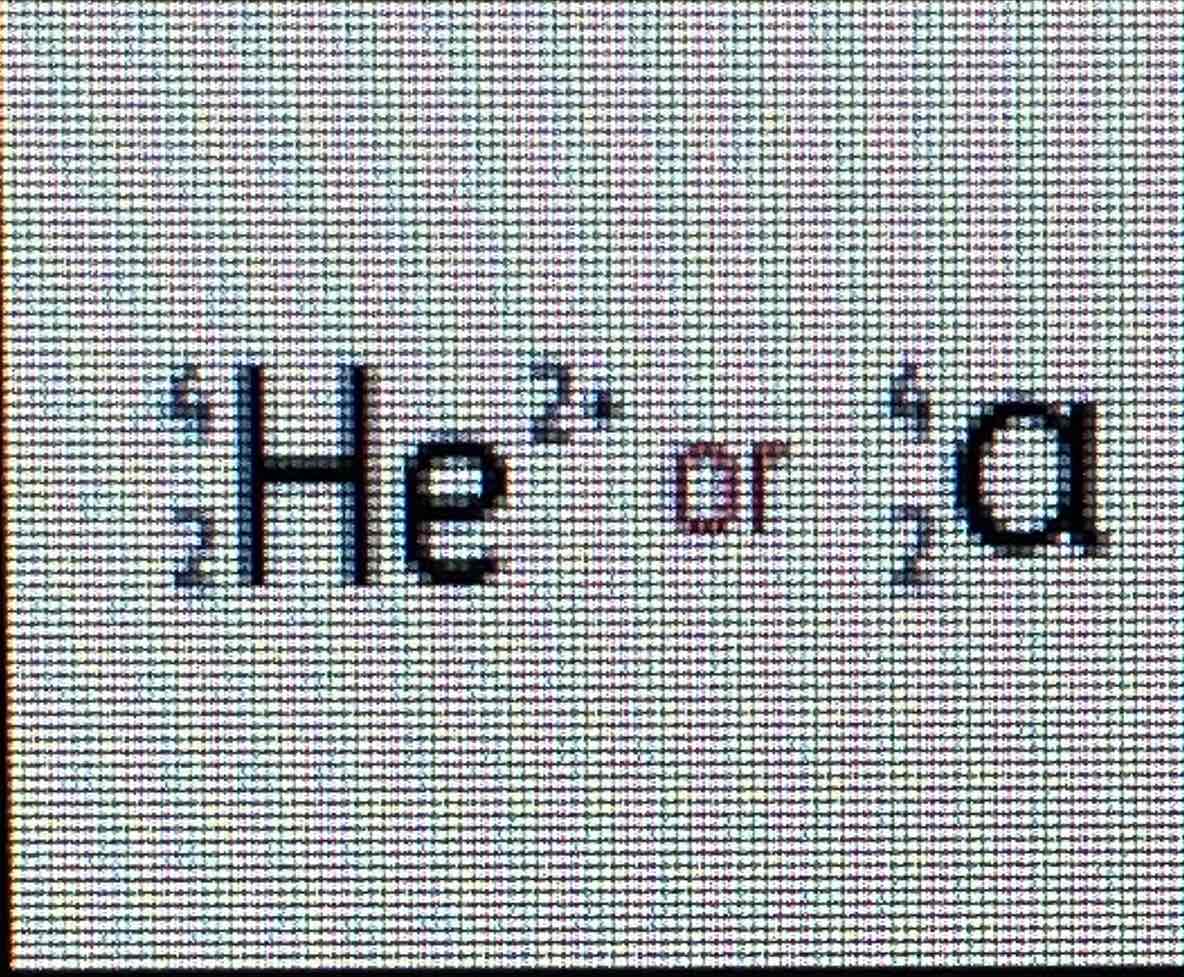
Effect of electric field on alpha emissions
Alpha particles are deflected towards the negatively charged plate
Effect of magnetic field on alpha emissions
Deflected in one direction
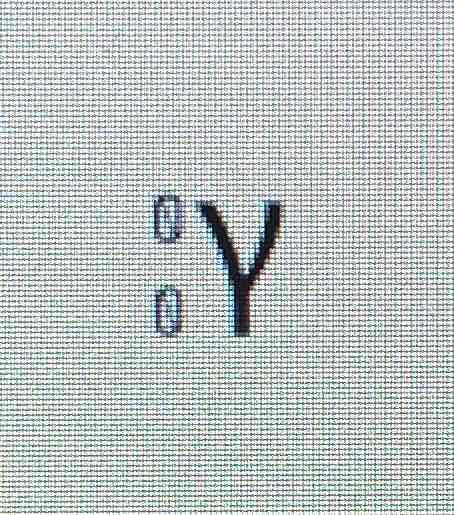
Beta particles
Fast moving electrons
Beta emissions
Streams of high energy electrons
More penetrating than alpha particles
Can travel through air and paper
Stopped by a thin layer of metal such as aluminium
Beta symbol

Effect of electric field on beta emissions
Beta particles are deflected towards the positive plate
Effect of magnetic field on beta emissions
Deflected in opposite direction to Alpha particles
Ionisation in radiation
when the types of radiation pass through matter, they knock electrons out of atoms, ionising them
Alpha = strongly ionising, transfer happens rapidly and so are the least penetrating
Gamma rays = weakly ionising, most penetrating of the radiations
Ionisation involves a transfer of energy from the radiation passing through the matter to the matter itself
Gamma rays
High energy electromagnetic radiation, and therefore no charge
Gamma emission
most penetrating of the three radiations
Can pass through several cm of lead or more than a m of concrete
Gamma symbol
Effect of electric field on gamma emissions
no effect
undeflected
Effect of magnetic field on gamma emissions
Unaffected
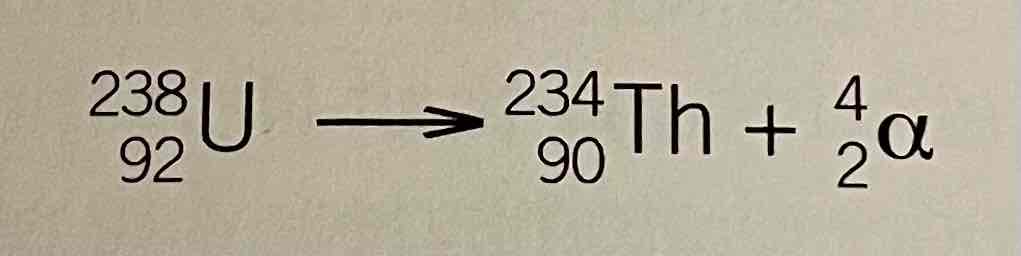
Effect of alpha emissions on mass number and atomic number
mass number decreases by 4
atomic number decreases by 2
Product is 2 places to the left in the periodic table
Effect of beta emissions on mass number and atomic number
mass number is unchanged
Atomic number increases by 1
Product is one place to the right in the periodic table
Effect of gamma radiation on mass number and atomic number
No effect as it’s a form of energy and is not an atomic particle
Alpha radiation equation example
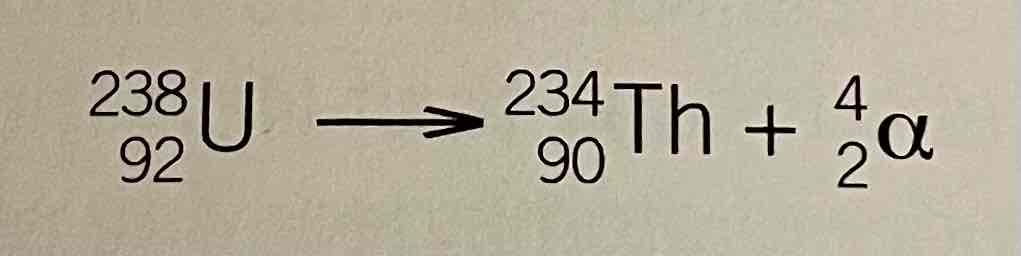
Beta- decay equation example
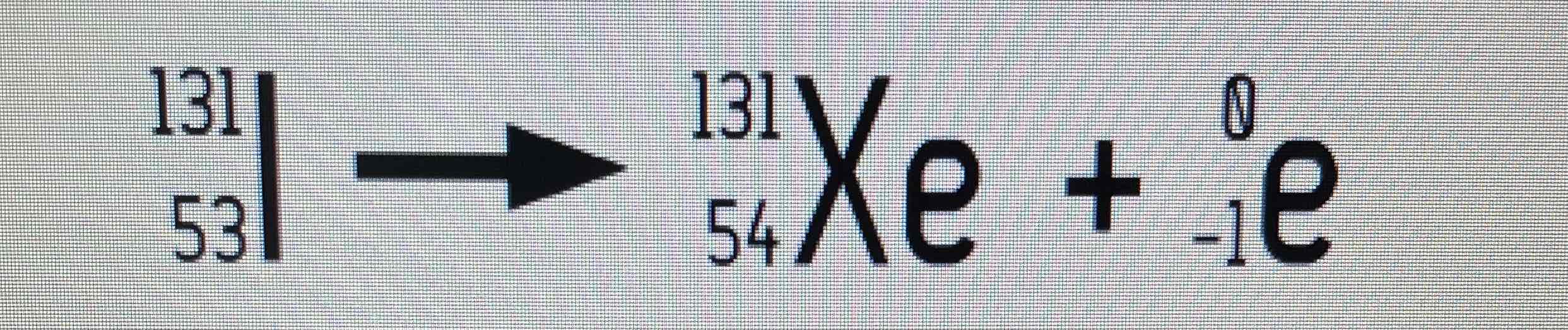
Electron capture
where one of the electrons is captured by a proton, turning it into a neutron
An electron neutrino is emitted (V_e)
Atomic number decreases by 1 due to the changing of the proton into a neutron
Electron is placed in reactants rather than products
Electron capture example equation
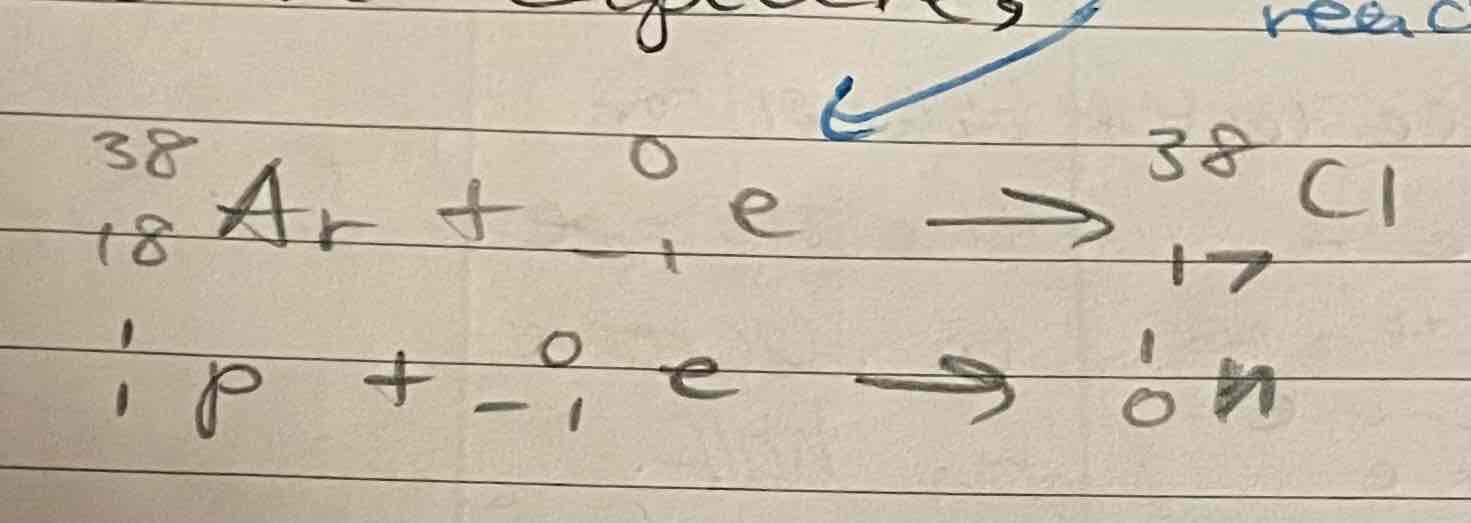
Positrons
produced by the splitting of a proton into a neutron and a positron
Type of B+ particle, this results in a decrease in atomic number
Reverse of beta emissions
Positron emission (B+ decay)
Atomic number decreases by 1
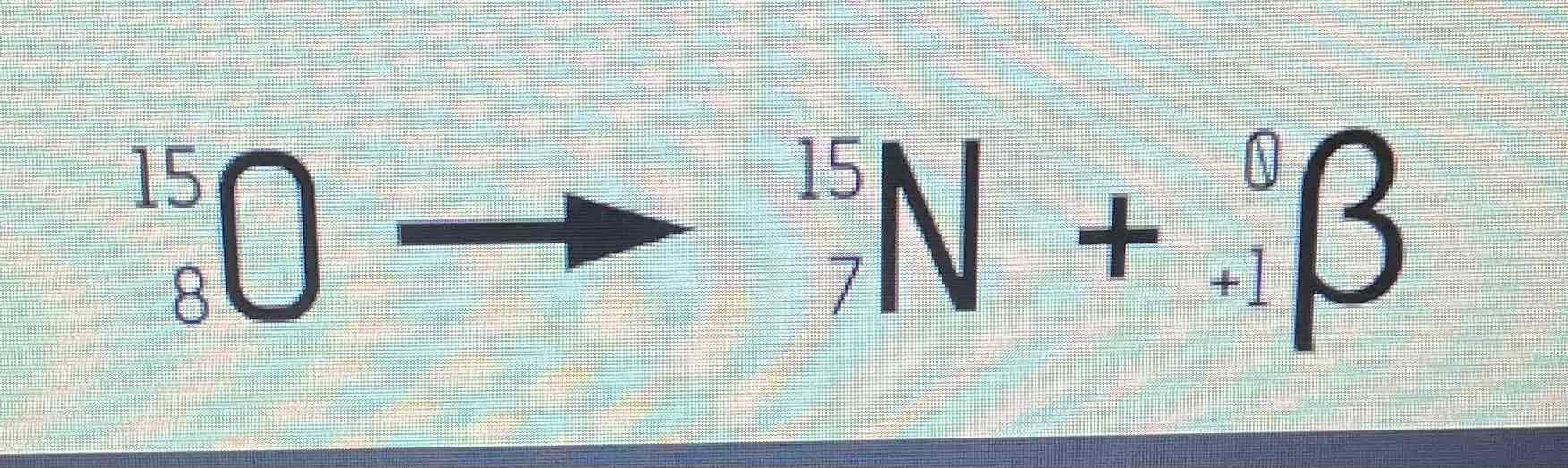
Positron emissions example equation
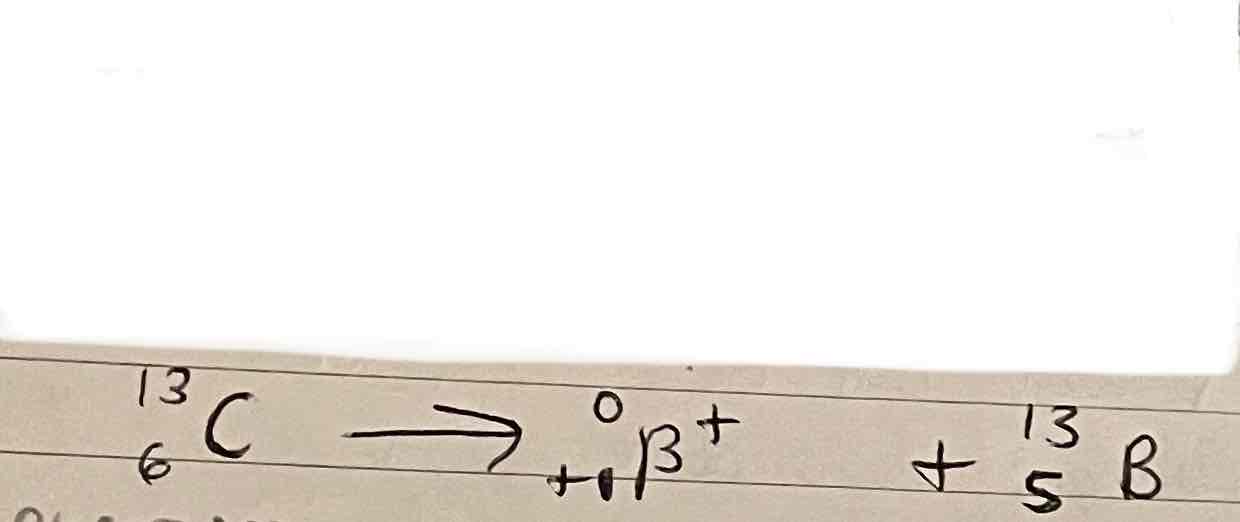
Half life
Time taken for half the atoms in a radioisotope to decay or the time taken for the radioactivity of a radioisotope to fall to half its initial value
Number of half life periods equations
Periods = time/half-life
Consequences of radiation on living cells
ionising radiation can damage the DNA of a cell;
damage to DNA may lead to changes in the way the cell functions, which can cause mutations and the formation of cancerous cells at lower doses or cell death at higher doses
outside the body, gamma radiation is the most hazardous
inside the body, if alpha particle emitting isotopes are ingested, they are far more dangerous than an equivalent of beta emitting or gamma emitting isotopes
Uses of radioisotopes in health and medicine

Uses of radio-isotopes in radio-dating
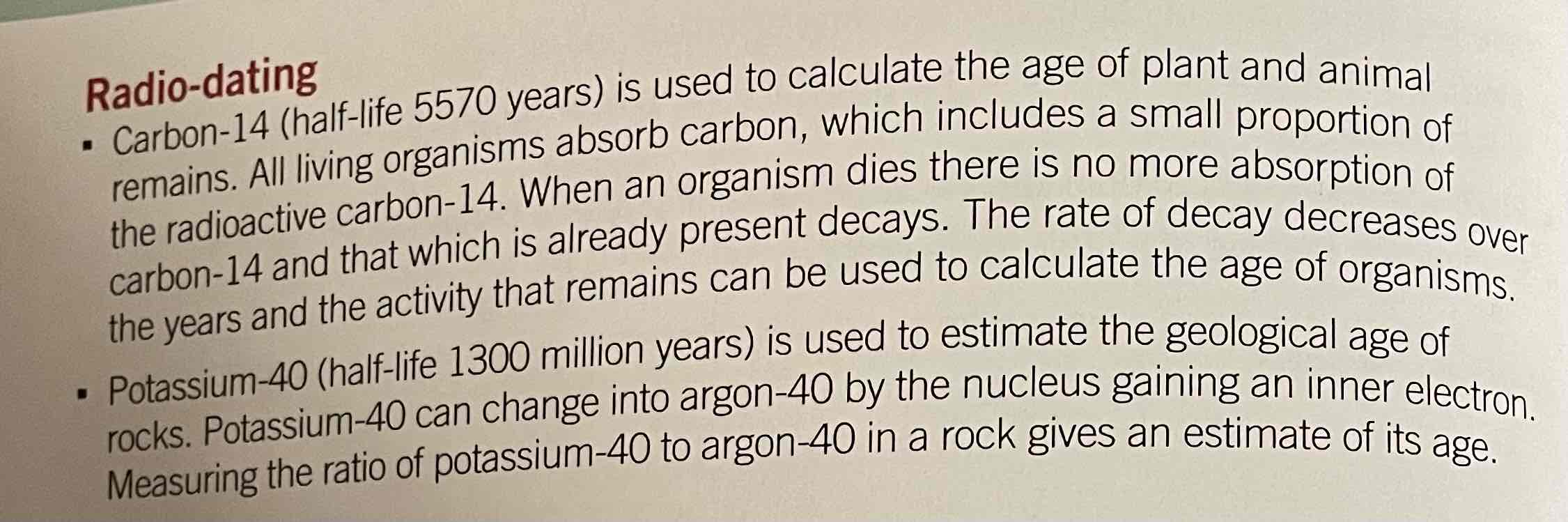
Uses of radioisotopes in industry and analysis

Radioactivity
caused by nuclear instability as a result of an unequal p:n ratio, causing the nuclei to change = radioactive decay
Optimum p:n = 1:1
Decay —> stable
Atomic orbital
A region in an atom that can hold up to two electrons with opposite spins
Fixed energy levels/quantum shells
electrons within atoms occupy fixed energy levels or quantum shells
Shells are numbered
The numbers are known as the principal quantum numbers, n
Lower value of n, closer the shell to the nucleus + lower the energy level
S orbital
spherical orbital
Represented by a sphere/circle
Holds up to 2 electrons
The 2 electrons have to be of opposite spin
P orbital
Dumbbell shaped
Hold up to 6 electrons
D orbital
can hold up to 10 electrons
Complex 3D shape
Electronic configuration
The arrangement of electrons in an atom
Filling shells and orbitals with electrons
electrons fill atomic orbitals in order of increasing energy
a maximum of 2 electrons can occupy any orbital each with opposite spins
The orbitals will first fill with one electron each with parallel spins, before a second electron is added with the paired spin
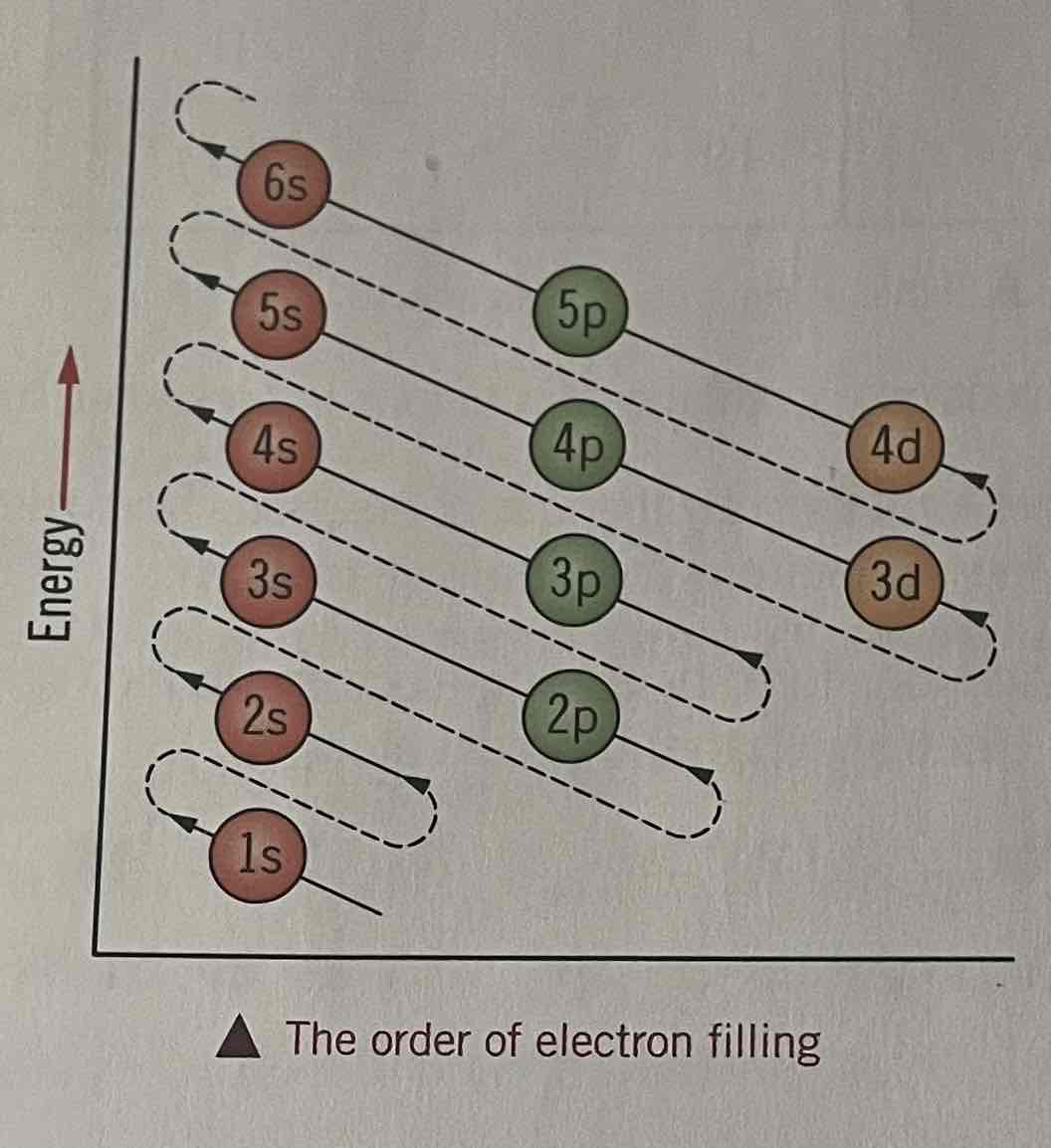
4s and 3d sub shells
4s is filled before 3d
Due to increasingly complex influences of nuclear attractions and electron repulsions upon individual electrons
The s orbitals have slightly lower energy than the d orbitals of the shells that are below them
Exceptions to the electron configuration rules
Config of;
chromium
Copper
Configuration of chromium

Configuration of copper
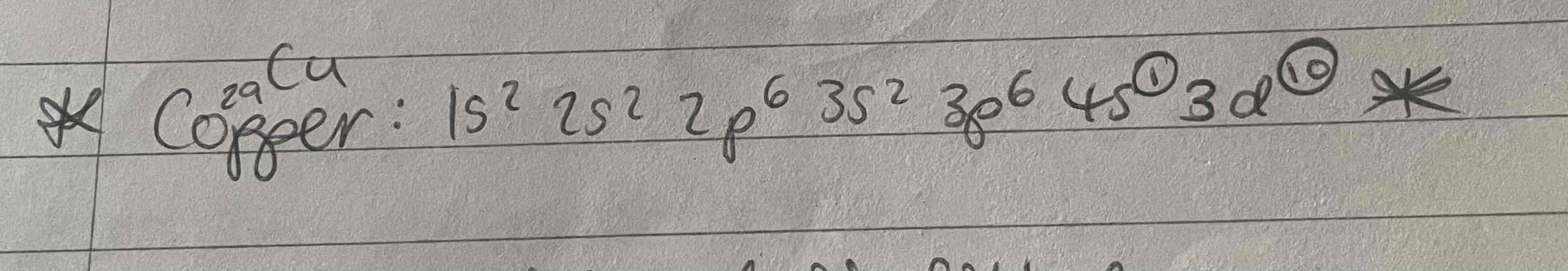
Ionisation
The process of removing electrons from an atom
The energy needed to remove each successive electron from an atom is called the first, second, third, second, etc ionisation energy
First standard molar ionisation energy
The energy required to remove one mole of electrons from one mole of gaseous atoms to produce one mole of gaseous ions. Under standard conditions, 25 degrees C and 1 atm
Equation of first ionisation energy
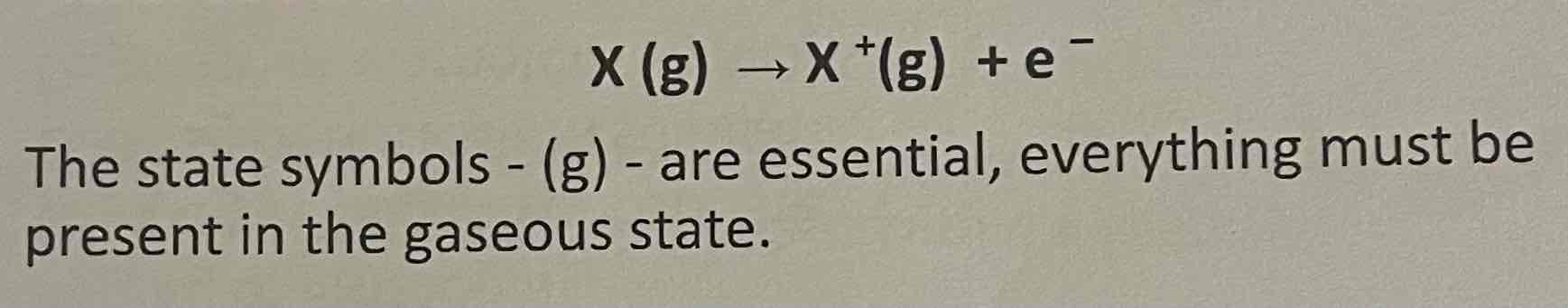
Factors affecting ionisation energy
The size of the positive nuclear charge
The distance of the outer electron from the nucleus
The shielding effect by electrons in filled inner shells
The effect of the size of the positive nuclear charge on ionisation energy
The greater the nuclear charge, the greater the attractive force on the outer electron and the greater the ionisation energy
The effect of the distance of the outer electron from the nucleus on ionisation energy
The force of attraction between the nucleus and the outer electron decreases as the distance between them increases. The further an electron is from the nucleus, the lower the ionisation energy
The effect of the shielding effect by electrons in filled inner shells on ionisation energy
Electrons in the filled inner shells repel electrons in the outer shell and reduce the effect of the positive nuclear charge. The more filled inner shells or subshells there are, the smaller the attractive force on the outer electron and the lower the ionisation energy
Patterns of ionisation energy across the periodic table
Steady increase across a period due to increasing nuclear charge with no extra shielding
Unexpected dips caused by different electron orbitals;
the dip between Mg and Al is caused by the p-electron being shielded by the full s-orbital
the dip between P and S is due to pairing of the p-electrons making the electron a little less stable (the half shell being more stable)
Shielding effect
The repulsion between electrons in different shells. Inner shell electrons repel outer shell electrons
The patterns in period 3 for ionisation energy
The first IE increase as you go across a period due to;
increasing nuclear charge (more protons in the nucleus)
the atoms have the same number of electron shells (same shielding)
The first IEs are lower for period 3 than period 2 due to the increased shielding of the extra full electron shell
Patterns of first ionisation energies graph
group 3 and 6 = anomalous (slightly lower IE than expected)

Successive ionisation energies
Are a measure of the energy needed to remove each electron in turn until all the electrons are removed from an atom
Explaining successive ionisation energies
The first electron is easiest to remove;
furthest from the nucleus + shielded by two full electron shells
The second electron shows a large jump
this electron is in a shell closer to the nucleus and there is less electron-electron repulsion
The next seven electrons all come from the same period and show a gradual increase in IE;
the p:e ratio is increasing so holds remaining electrons are held more tightly
greater effective nuclear charge as the same number of protons are holding fewer and fewer electrons
as each electron is removed, there is less electron-electron shielding and so each shell is drawn in slightly closer to the nucleus
the distance of the electron from the nucleus decreases, the nuclear attraction increases
The final two electrons require much more energy;
they are in the electron shell closest to the nucleus
there is much stronger nuclear attraction and no shielding
Equations of successive ionisation energies
Charge of ion made increases by +1
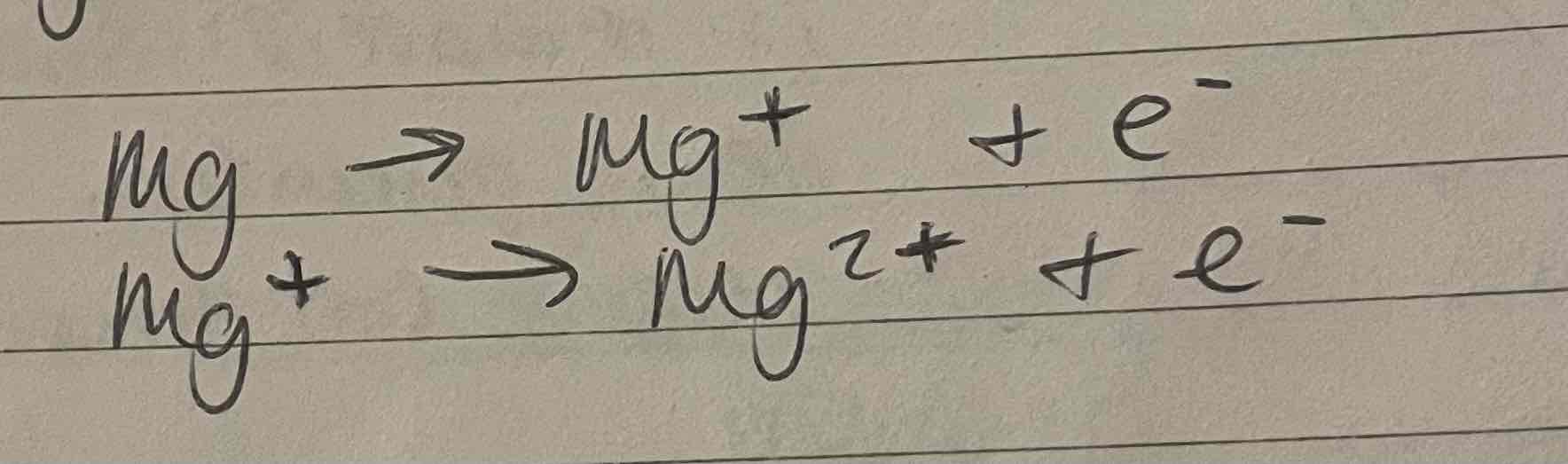
Relationship between energy and frequency
E=hf
Energy = planck’s constant x frequency
Relationship between frequency in wavelength
f=c/wavelength
Frequency=speed of light/wavelength
Emission spectra
Coloured lines on black background
each of the lines on the emission spectra represents a drop from one energy level down to another
energy levels converge at higher energies
most common transition; n=2 —> n=1
atoms are given energy, electrons are excited and the additional energy promotes then from a lower energy level to higher one
When the source of energy is removed and the electrons leave the excited state, they fall from the higher energy level to a lower energy level
Energy lost is released as a photon (a quantum of light energy) with a specific frequency
Absorption spectra
lines represent electron promotions
black lines on coloured backgrounds
When white light is passed through the vapour of an element, certain wavelengths will be absorbed by the atoms and removed from the light
Black lines appear in the spectrum where light of some wavelengths has been absorbed
The wavelengths of these lines correspond to the energy taken in by the atoms to promoting electrons from lower to higher energy levels
Atomic emission spectrum of the hydrogen atom
Consists of seperate series of lines mainly in the ultraviolet, visible and infrared regions of the em spectrum
Atom is excited by absorbing energy, an electron jumps to a higher energy level. Electron falls back down to a lower level, it emits energy in the form of EM radiation. This emitted energy = line in the spectrum as energy of emitted radiation = difference between the two energy levels
E=hf therefore electronic transitions between different energy levels = emission of radiation of diff freq + therefore producing different lines in the spectrum
Energy, frequency and wavelength of spectrum
Left —> right;
energy increases
frequency decreases
wavelength increases
as frequency increases, the lines get closer together as energy difference between the shells decreases
Lyman series
Ultraviolet region
Transitions back to first shell/n=1 energy level (ground level)
As frequency increases, the number of the lines on the spectrum also increases
Highest frequency
Highest energy
Most common transition = n=1–>n=2
Balmer series
visible region
Drops back to the n=2 region
Frequency increases
Wavelength increases
1 less line than Lyman series
Paschen series
Infrared region
Drops back to the n=3 level
As frequency decreases, the number of lines also decreases
Convergence limit
When the spectral lines become so close together they have a continuous band of radiation and seperate lines cannot be distinguished
Representing IE on an energy diagram
Arrow from n=1–>n=infinity
Relationship between the frequency of the convergence limit of the Lyman series and the IE of the hydrogen atom
convergence limit represents the ionisation of the hydrogen atom
Measuring the convergent frequency (n=1–>n=infinity) allows the IE to be calculated using E=hf