General Chemistry
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Definition of ‘mole’ (mol)
Unit of measure; describing the amount of a chemical substance that contains as many atoms, formula units, or molecules as there are in exactly 12 grams of pure Carbon (¹²C).
Relationship between Moles and Avogadro's Number
One mole of a substance has 6.022×10²³ atoms
Avogadro's Number =
6.022×10²³
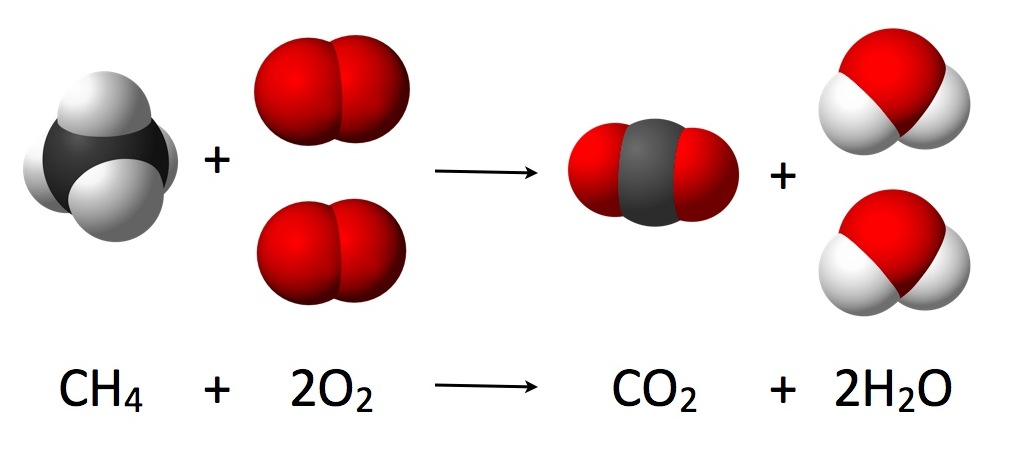
Stoichiometry can be used to ______
To determine how much of each reactant is required for all reactants to be used up at the same time
What is matter?
Anything that occupies space and has mass.
How can matter be classified?
Matter can be classified based on its state (solid, liquid, gas) and its composition (elements, compounds, mixtures).
What are the states of matter?
The states of matter are solid, liquid, and gas. Each state exhibits different properties and behaviors.
Define an atom
An atom is a submicroscopic particle that constitutes the fundamental building blocks of ordinary matter.
What is a molecule?
A molecule is formed when two or more atoms bind together in specific geometrical arrangements.
What determines the properties of matter?
The properties of matter are determined by the properties of molecules and atoms.
What is the scientific method?
The scientific method is a process for understanding nature through observation, hypothesis formulation, experimentation, and the formulation of laws and theories.
What is a hypothesis?
A hypothesis is a tentative interpretation or explanation of observations that can be tested by experiments.
What is a scientific law?
A scientific law is a brief statement that summarizes past observations and predicts future ones, such as the law of conservation of mass.
How is a scientific theory defined?
A scientific theory is a model that explains the way nature operates, based on well-stablished hypotheses validated by experiments.