Periodic Trends
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Mendeleev(1869)
organized the elements by increasing atomic mass and so that elements in the same row have similar properties
Moseley(1913)
rearranged the elements by increasing atomic number
this is how the modern periodic table is arranged today
Periodic Law
When elements are arranged in order of increasing atomic number, there is a periodic pattern in their physical and chemical properties
Period
Group
horizontal rows of the table
vertical columns on the table
In the same group
similar properties
numbered 1-18
In the same period
doesn’t not have similar properties
numbered 1-7
same number of occupied energy levels
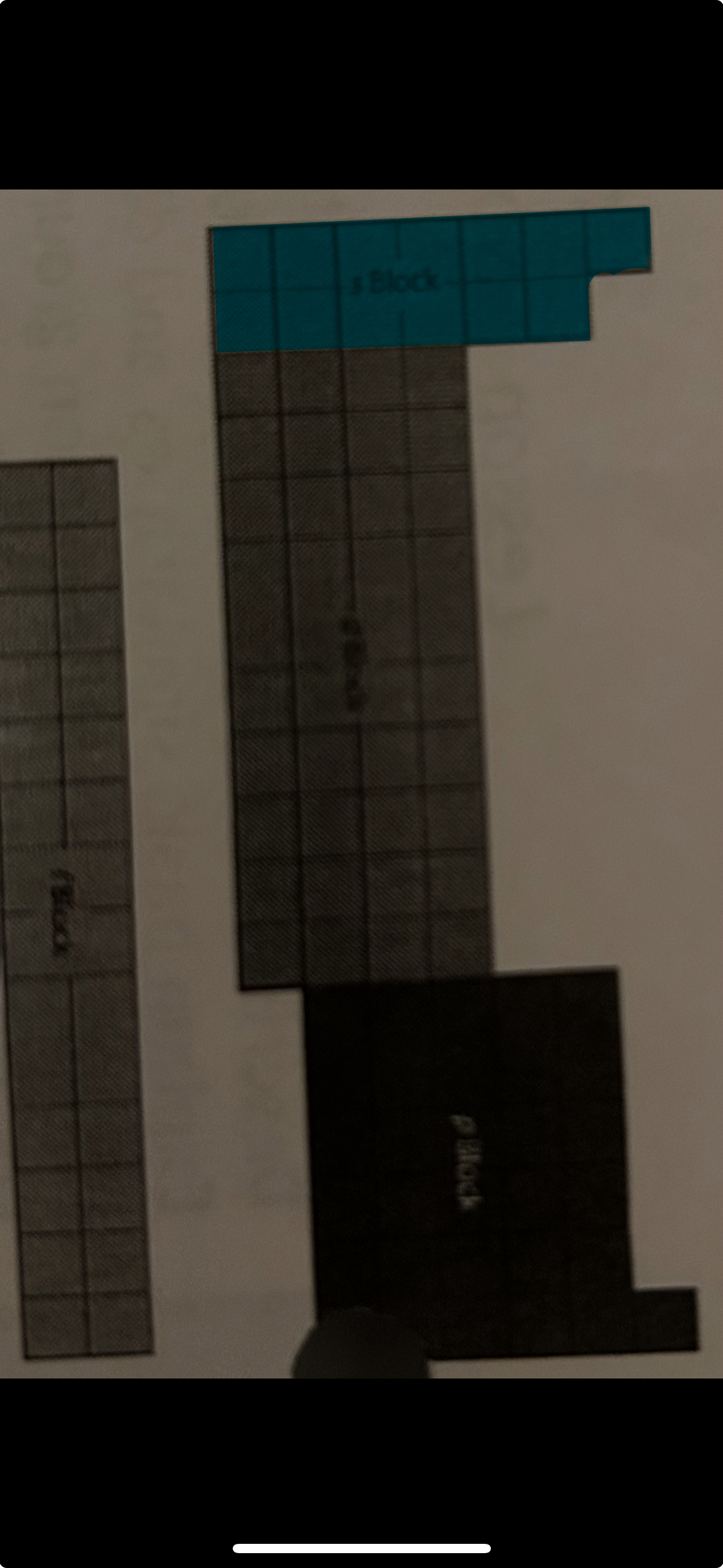
s block
representative elements

p block
representative elements
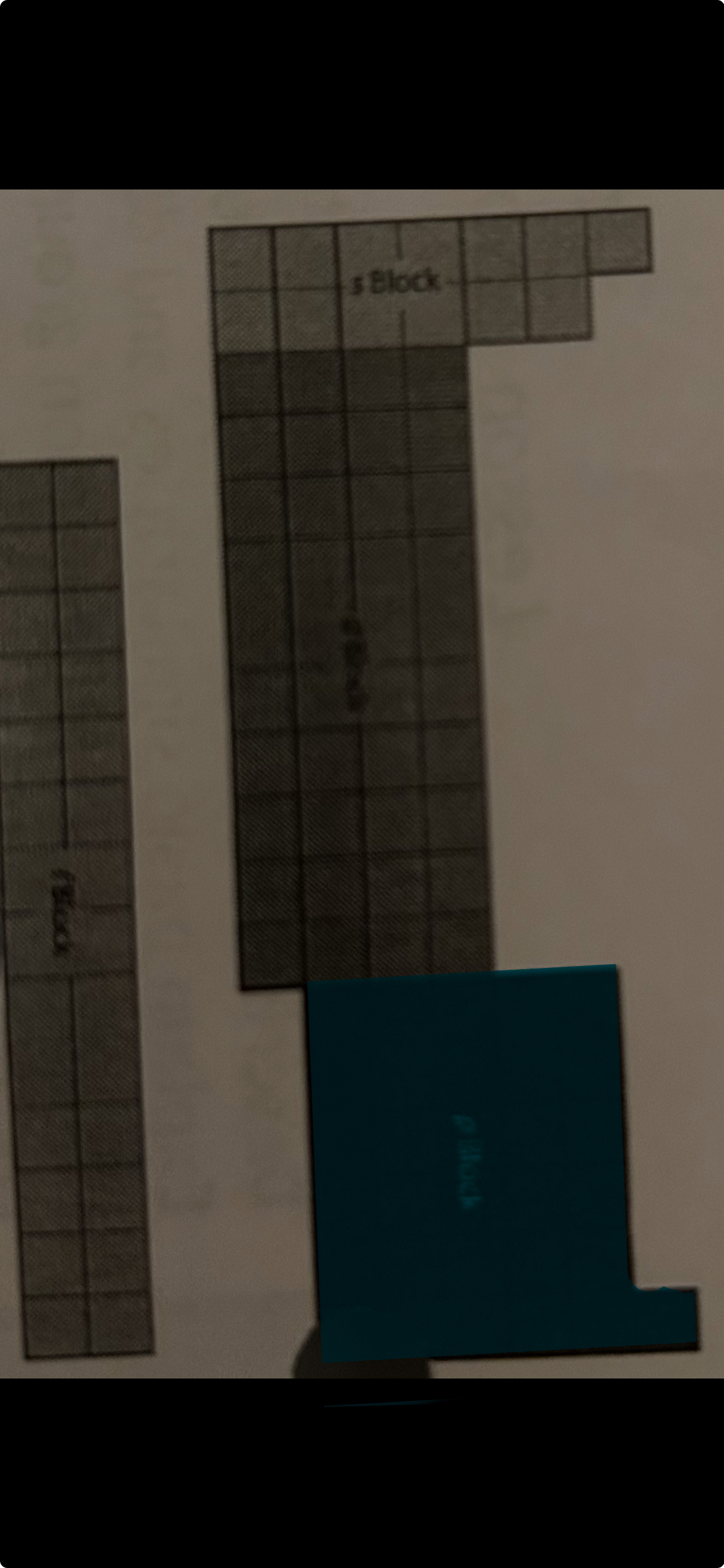
d block
transition metals

f block
inner tradition metals
Metals
left of staircase
lustrous(shiny)
malleable: hammer into thin sheets
ductile: make into thin wire
good conductors of heat and electricity
Nonmetals
right side of the staircase(hydrogen included)
non-lustrous(dull)
brittle: breaks easily
poor conductors good insulators
Metalloids
Touch the staircase and have properties of both metals and nonmetals with the exception of aluminum. It is a metal.
they are semi conductors, which means they normally do not conduct electricity, but will conduct at high temperatures or when certain substances are added
Atomic Radius
An atom does not have a fixed radius. The radius can only be found by measuring between two nuclei of two touching atoms then halving that distance.
½ of the distance between the nuclei of two like atoms
it is dependent on nuclear charge(magnitude of attraction from nucleus) and distance between electrons and nucleus
Period Trend
Group Trend
decreases left to right—greater number of protons
increases down—greater number of occupied energy levels
Shielding Effect
the inner electrons shield or block the attraction from the nucleus.
while, there is not an increase in number of protons down a group. This does not affect the magnitude of attraction from the nucleus due to this effect.