oxidation of alcohols
0.0(0)
0.0(0)
Card Sorting
1/7
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
8 Terms
1
New cards
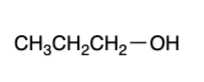
primary alcohol — “mild” oxidizers
aldehyde
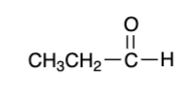
2
New cards
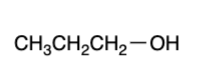
primary alcohol — “strong” oxidizer
carboxylic acid
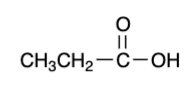
3
New cards
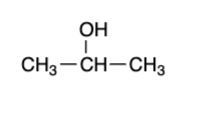
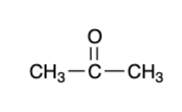
secondary alcohol — “mild” oxidizers
ketone
4
New cards
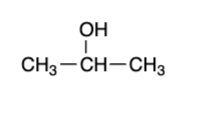
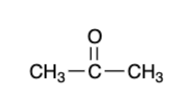
secondary alcohol — “strong” oxidizer
ketone
5
New cards
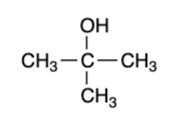
tertiary alcohol — “mild” oxidizers
no reaction
6
New cards
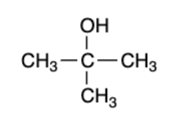
tertiary alcohol — “strong” oxidizer
no reaction
7
New cards
“mild” oxidizers
PCC, CH2Cl2
NaOCl, CH3CO2H, 0 C
Swern Oxidation:
(CH3)2SO, (COCl)2, -60 C
Et3N
8
New cards
“strong” oxidizer
H2CrO4 (Na2Cr2O7, H2SO4 sometimes called “Jones Reagent” or “Jones Oxidation”)