ch 3: chemical signalling in the NS
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
the basis of neuronal communication (3)
communication btw neurons occurs at synapses via chemical NTs that cross the synaptic cleft
transmission is one-way: pre → postsynaptic cell
NT contained in synaptic vesicles
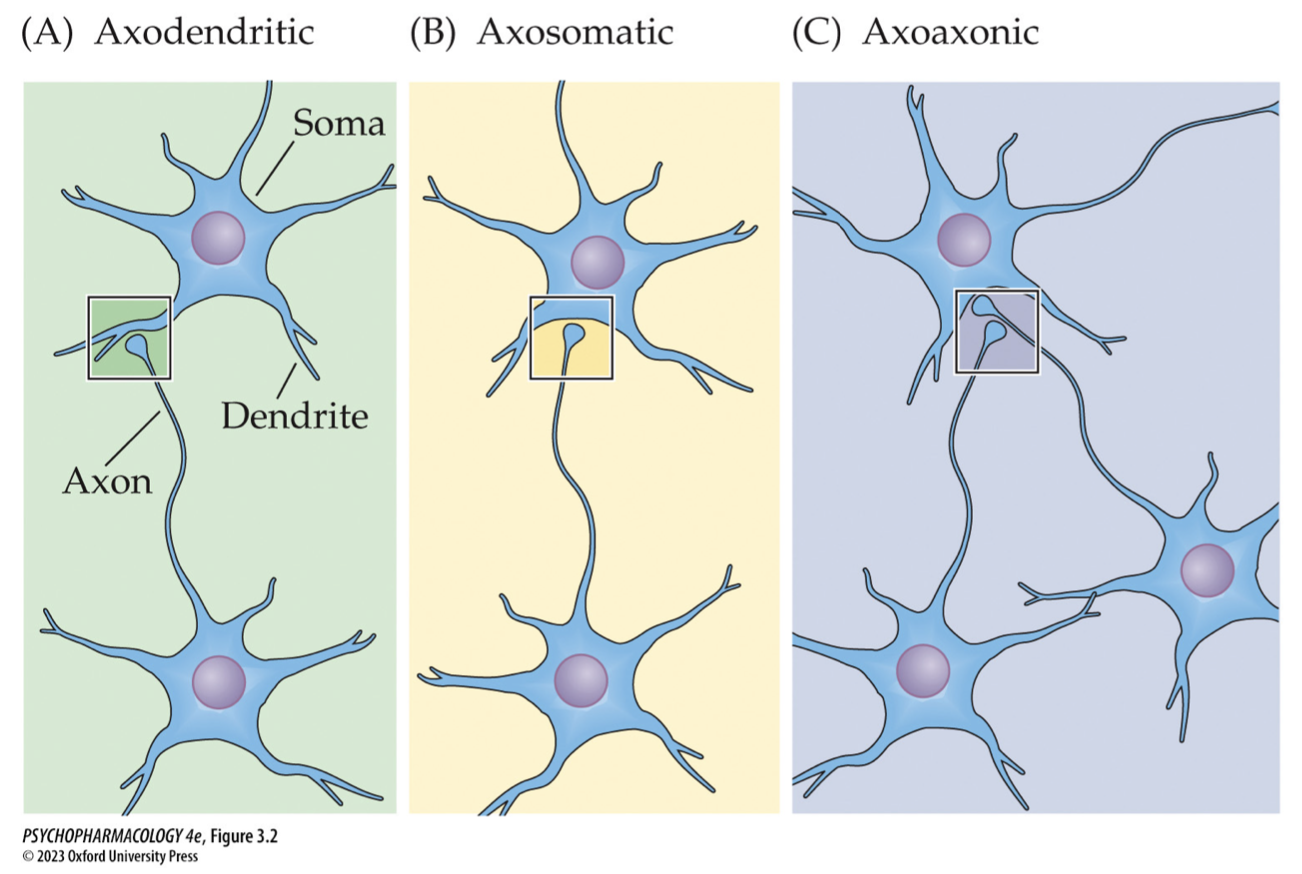
types of synapses (6)
axodendritic: axon to dendrite
axosomatic: axon to cell body
axoaxonic: axon to axon
neuromuscular junction: axon to muscle
electrical: electric current flows along specialized proteins → mediated by ∆s in membrane potential
mixed: both chemical + electrical transmission → release of NT bc of ∆ in voltage
criteria that makes a NT (4)
must be in brain
manufactured in synapse
has to be present + then removed when not needed → has to bbe specific signal
has to signal through some mechanism
classes of NT (5)
amino acids + monoamines
acetylcholine (ACh)
ATP and adenosine
neuropeptides, lipids, gases
elements → ie. Zinc but not manufactured
how is the synthesis of neuropeptides different from other NTs
most NT synthesized in axon terminals
NPs are synthesized from precursor proteins that are synthesized in the cell body + shipped to the axon terminals
replenishment of NPs is slower than for small-molecule NTs
neuromodulators (3)
alter the action of standard NTs
diffuse away frim the site of release to influence more distant cells → volume transmission
some transmitters may act in both ways
classical NT release triggered by ___
triggered by Ca2+ influx at membrane depolarization
Ca2+ mediates release of transmitter from vesicles by exocytosis
define exocytosis (3)
the fusion of the vesicle membrane the membrane of the axon terminal
exposes the inside of the vesicle to the outside of the cell
vesicle is opened + NT molecules are allowed to diffuse into the synaptic cleft
what are active zones (3)
specialized regions near the postsynaptic cell where NT occurs
stain darkly on the electron micrograph
vesicle must be transported to an active zone for exocytosis to occur
three models of vesicle recycling
clathrin-mediated endocytosis
ultrafast endocytosis
kiss-and-run
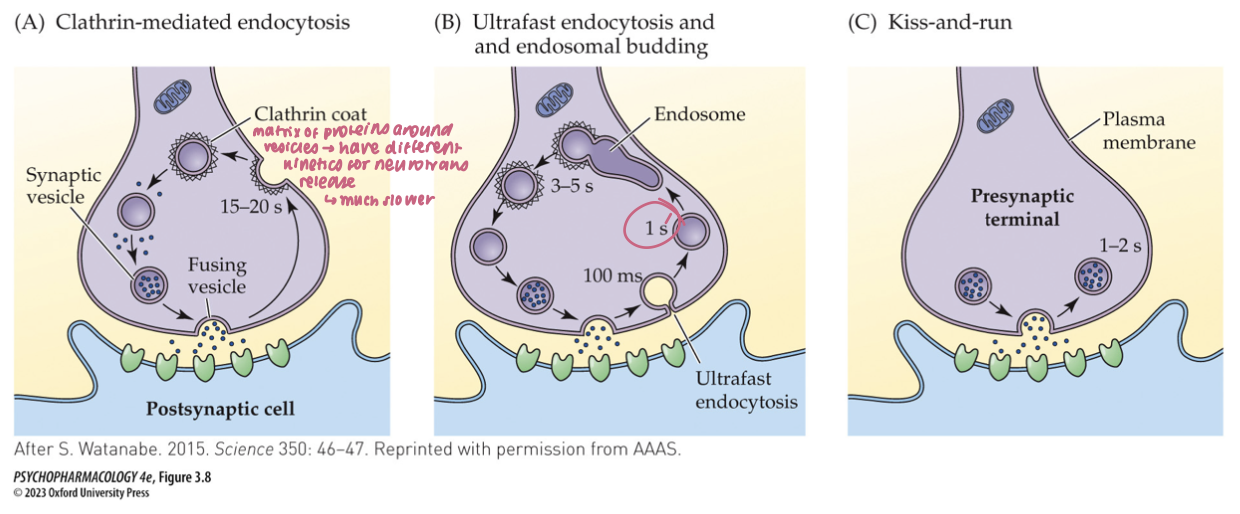
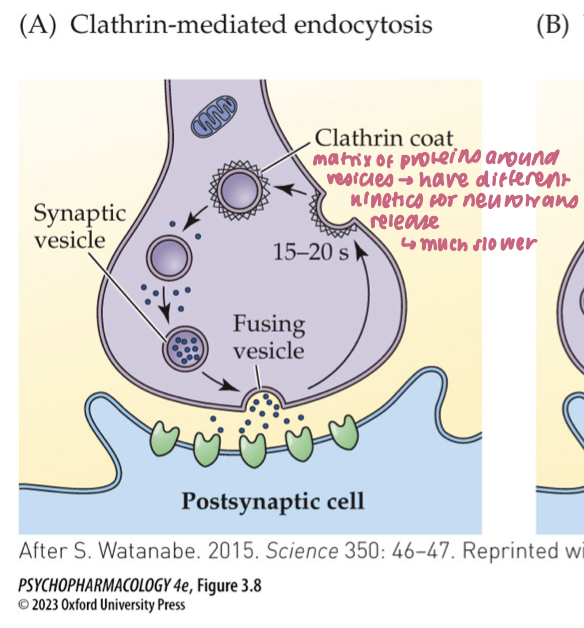
clathrin-mediated endocytosis (3)
After full-collapse fusion, vesicle membrane diffuses away from the release site
Clathrin + adaptor proteins coat the membrane; dynamin pinches it off → new vesicle
Time scale: ~10–20 s; supports low–moderate activity
🧠 Takeaway: Full collapse → clathrin retrieval away from the active zone.
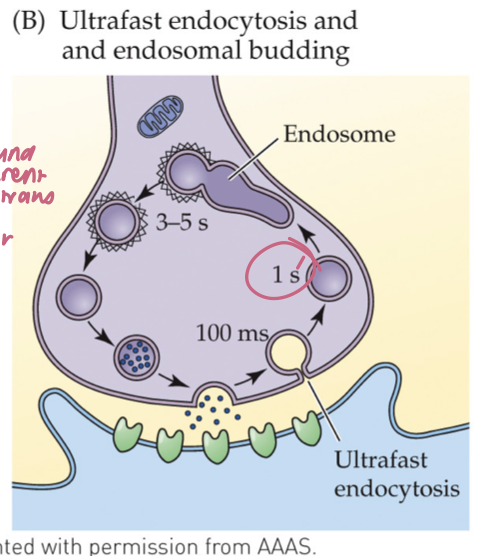
ultrafast endocytosis (3)
Within ~50–100 ms of fusion, membrane is rapidly internalized near the active zone (no clathrin yet)
Internalized membrane forms an endosome; clathrin is used later to bud new synaptic vesicles from the endosome
Operates under typical activity; faster local retrieval than classical clathrin
🧠 Takeaway: Grab fast first (no clathrin), then rebuild vesicles from an endosome with clathrin.
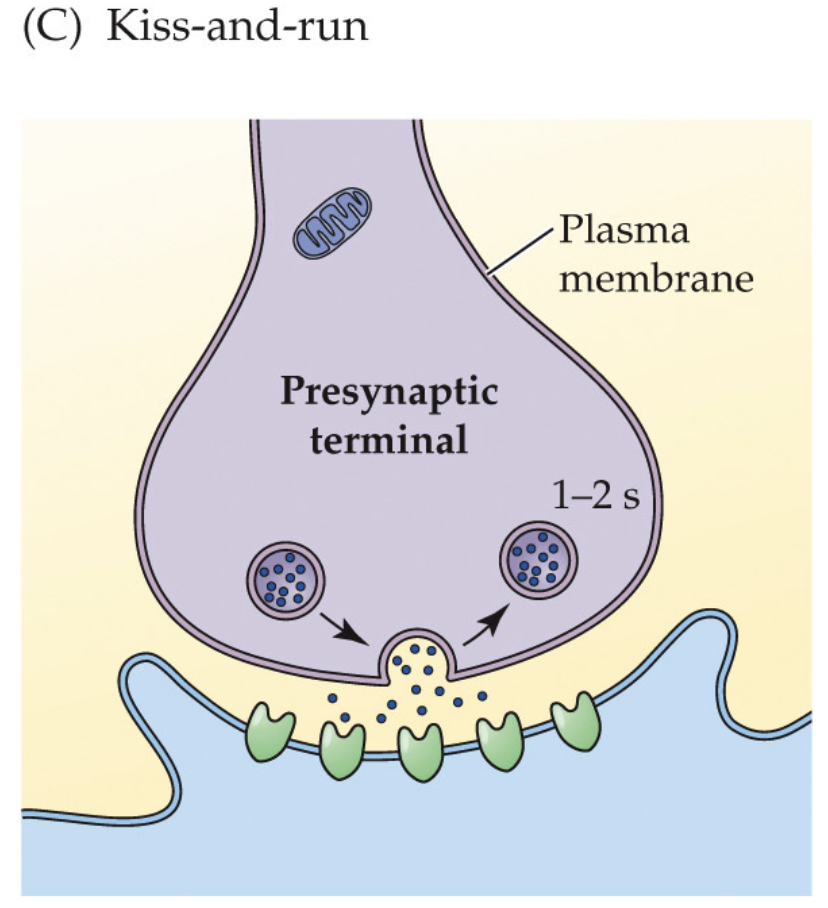
kiss-and-run (3)
Vesicle makes a transient fusion pore, releases transmitter, then reseals without full collapse
Local, rapid reuse; clathrin not required for retrieval
Minimizes mixing of vesicle/plasma membranes; evidence mixed/controversial
🧠 Takeaway: Brief pore, quick reseal—vesicle “kisses” the membrane and “runs.”
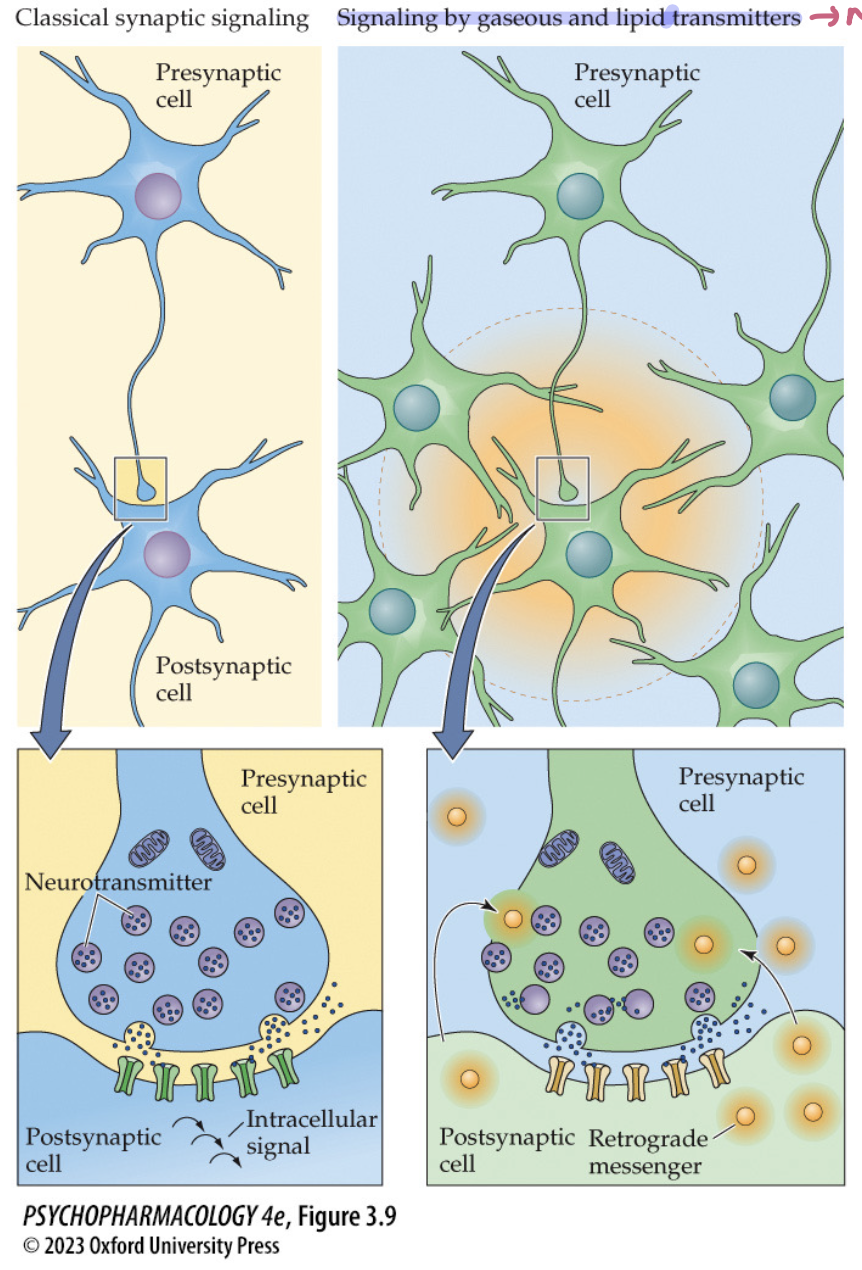
what sets lipid + gaseous NTs apart from classic NTs? (3)
not stored in vesicles
synthesized on demand by postsynaptic cell after receptor activation by a classical NT
act as retrograde messengers on the presynaptic cell + also diffuse to other neurons
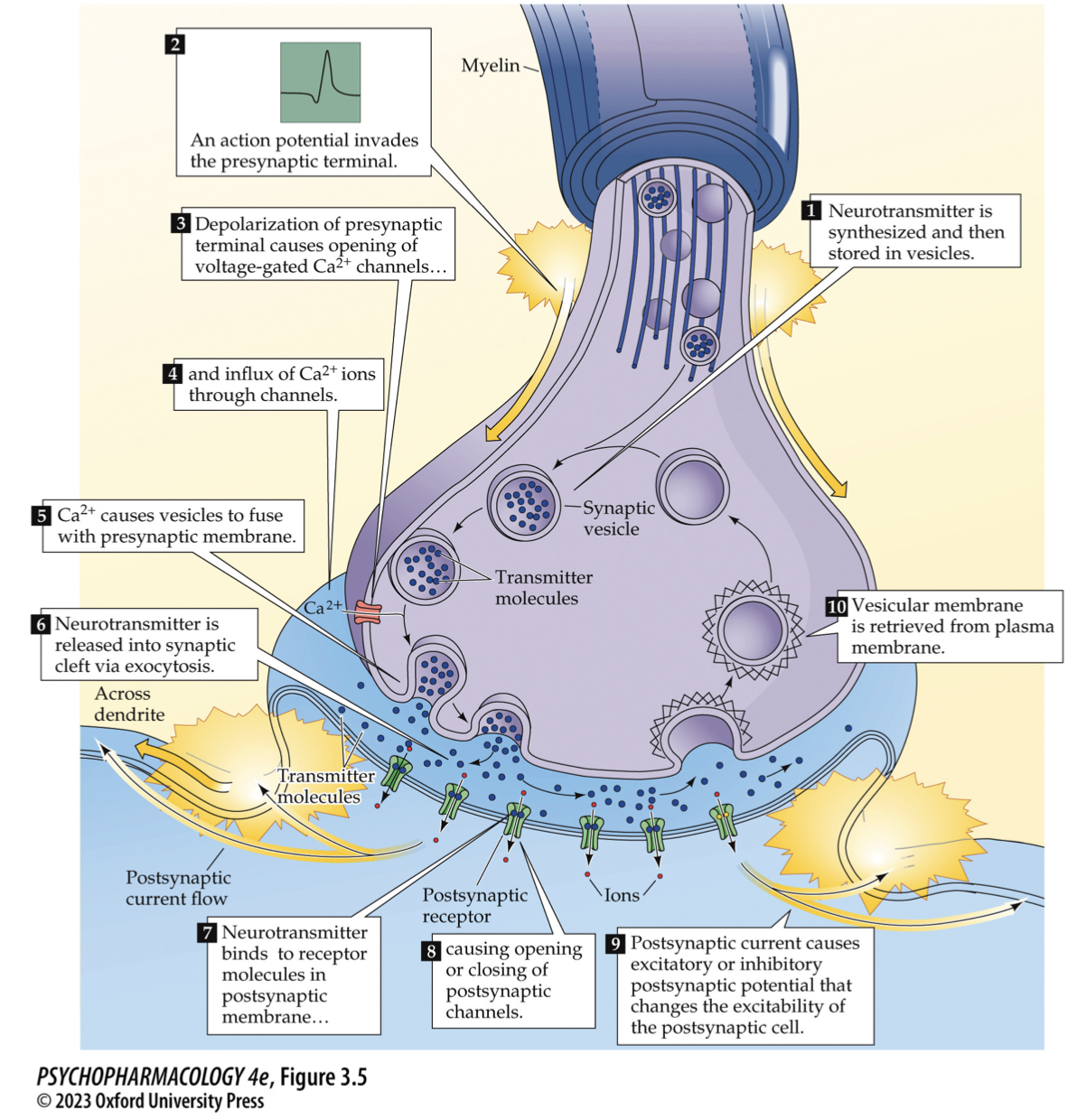
Classical synaptic transmission — step map (draw it)
AP arrives → presynaptic depolarization → voltage-gated Ca²⁺ channels open → Ca²⁺ influx
SNARE-primed vesicle fuses (Ca²⁺ sensor = synaptotagmin) → NT released
NT binds ionotropic (fast) or metabotropic (GPCR) receptors → EPSP/IPSP
Termination: reuptake (transporters), enzymatic breakdown, or diffusion; membrane retrieved by endocytosis
🧠 Takeaway: Ca²⁺ + SNAREs → fusion; transporters/enzymes → signal off.
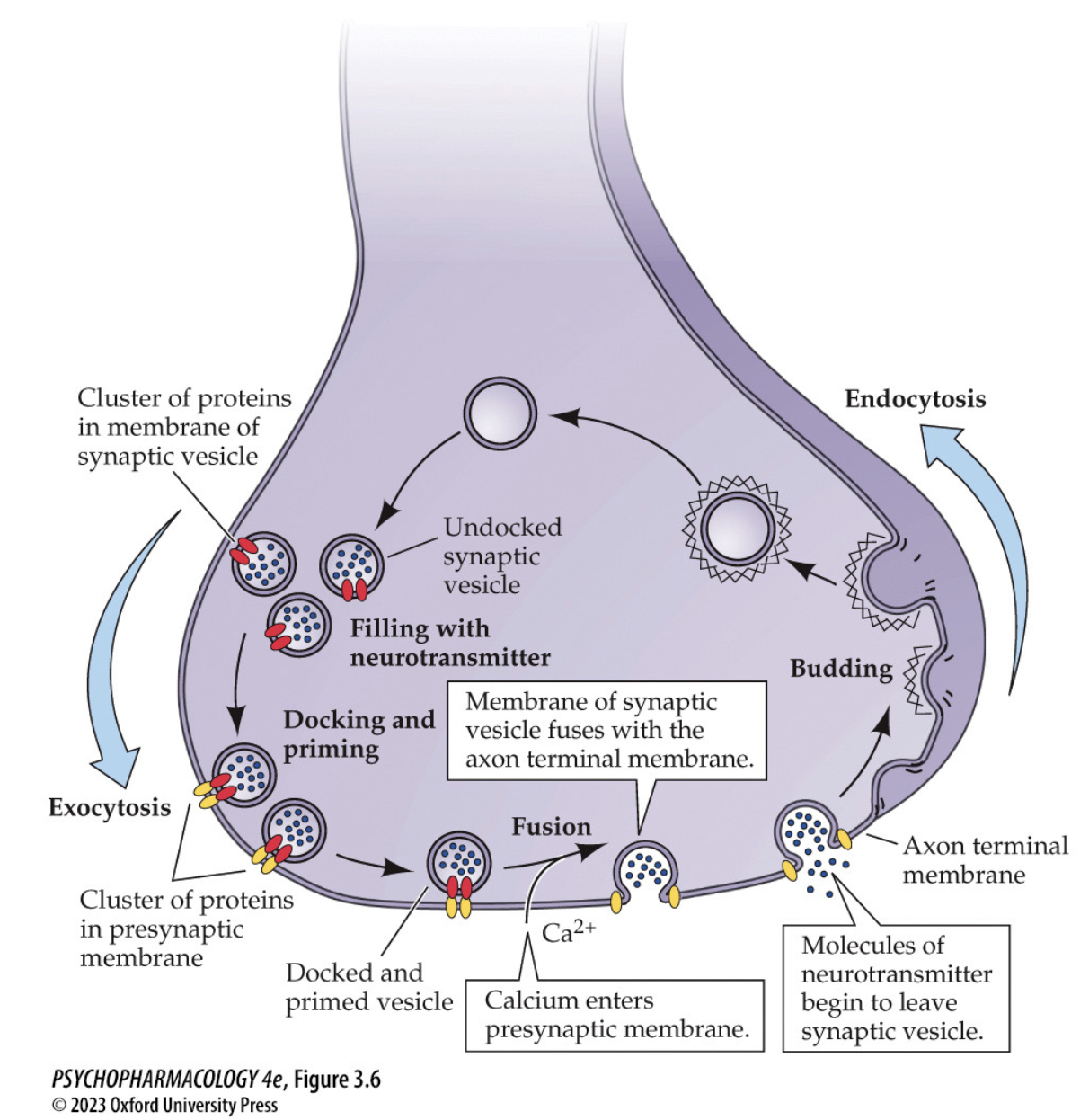
Synaptic vesicle cycle — reload & recycle (draw it)
Endocytosis → budding of new vesicle → filled by vesicular transporters
Docking & priming at active zone (SNARE complex assembled)
Ca²⁺ entry triggers synaptotagmin-mediated fusion → exocytosis
Membrane recycled (clathrin pathways) → refill → repeat
🧠 Takeaway: Dock–prime–Ca²⁺ trigger–fuse–recycle = the loop that keeps release going.
mechanisms that control the rate of NT release by nerve cells (3)
rate of cell firing → how quickly an AP invades the terminal
(more APs → more Ca²⁺ entries → ↑ release)
probability of NT release → synapses vary in the probability that vesicles will undergo exocytosis
at the terminal (synapse-specific; # of docked/primed vesicles, Ca²⁺ coupling)
presence of autoreceptors → (usually ↓ further release)
3 kinds of autoreceptors
terminal: inhibit further transmitter release
somatodendritic: slow the rate of cell firing
heteroreceptors: receive transmitters at axoaxonic synapses; either enhance or reduce transmitter release
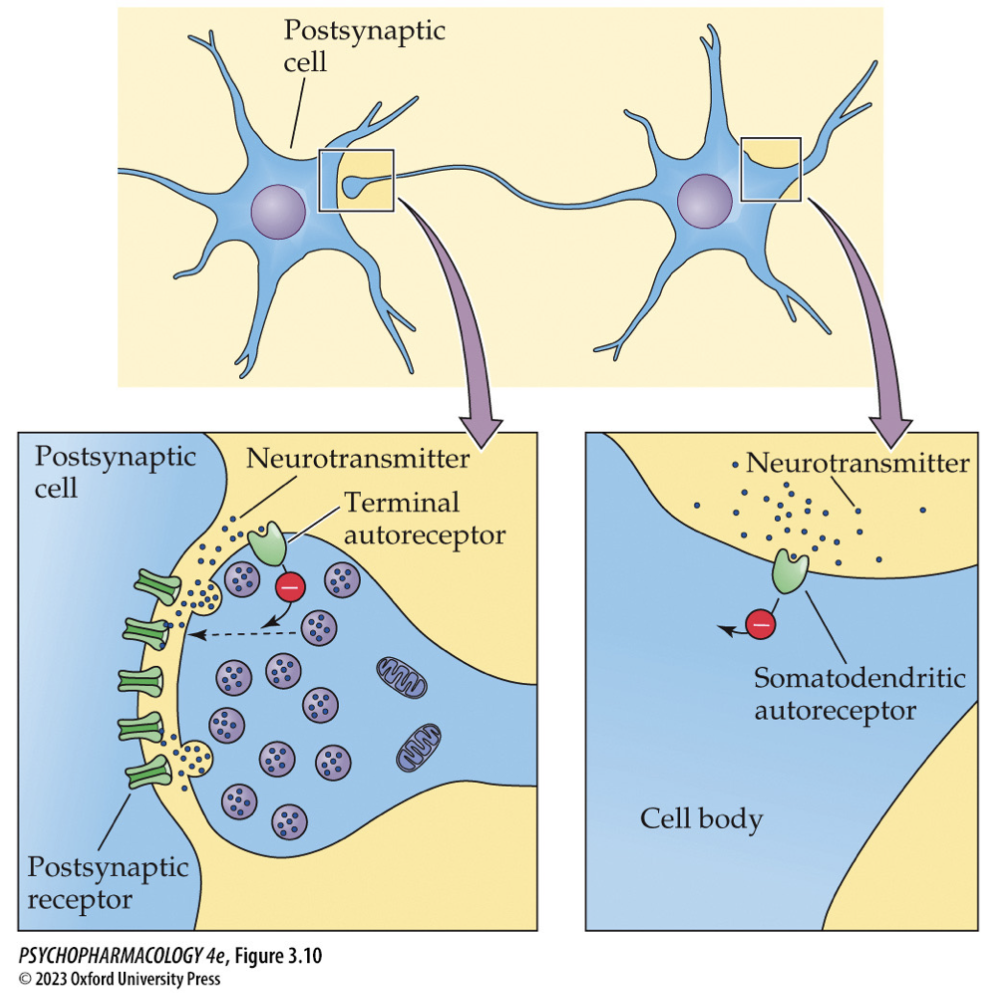
Terminal autoreceptors — function & mechanism (3)
Located on presynaptic terminal
Activated by the neuron’s own transmitter
Inhibit further release/synthesis (negative feedback; often via ↓ Ca²⁺ entry or ↑ K⁺)
🧠 Takeaway: Terminal autoR = “we’ve released enough—slow down.”
Somatodendritic autoreceptors — how are they different? (2)
Located on cell body/dendrites of the same neuron
Activation reduces firing rate (hyperpolarization), which indirectly lowers release at terminals
🧠 Takeaway: Somato-dendritic autoR = turn down the pacemaker.
Heteroreceptors (axoaxonic control) — what do they do? (3)
Presynaptic receptors activated by another neuron’s transmitter (not its own)
Found at axoaxonic synapses
Can decrease release (e.g., presynaptic inhibition via Gi GPCRs) or increase release (facilitation)
🧠 Takeaway: Neighboring axons can dial your release down or up.
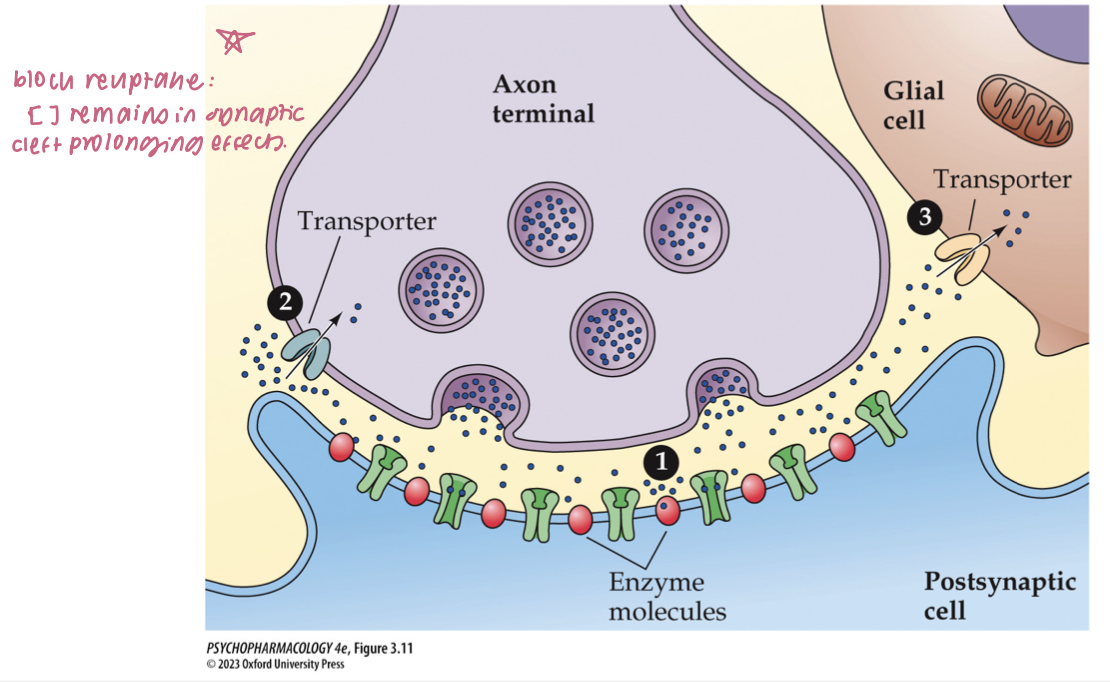
mechanisms of NT inactivation (3)
enzymatic breakdown w/in or near the synaptic cleft
reuptake: removal from synaptic cleft by transporter proteins on the axon terminal membrane
uptake by postsynaptic cell or glial cells
Neurotransmitters outside the CNS — what to know (3)
Many of the same transmitters exist inside & outside CNS
Some are made by gut bacteria; drugs can alter the microbiome
🧠 Takeaway: Don’t think “brain-only”—transmitters act system-wide.
The gut–brain axis (bidirectional) (3)
Two-way signaling between gut (incl. microbiome) and brain
Pathways: vagus nerve + systemic routes (immune, endocrine, metabolites)
🧠 Takeaway: Gut activity can change brain function and vice versa.
Neurotransmitter receptors (4)
Proteins on plasma membranes (neurons, muscle, secretory cells)
Ligand binding → conformational change → response in the target cell
Responses can be excitatory or inhibitory
🧠 Takeaway: Receptors translate chemical binding into cellular action.
Receptor subtypes (same NT, different outcomes) (5)
A single NT has multiple receptor subtypes with distinct signaling
Different subtypes ⇒ different effects & drug selectivity
ionotropic
metabotropic
🧠 Takeaway: Which subtype is hit matters as much as which transmitter.
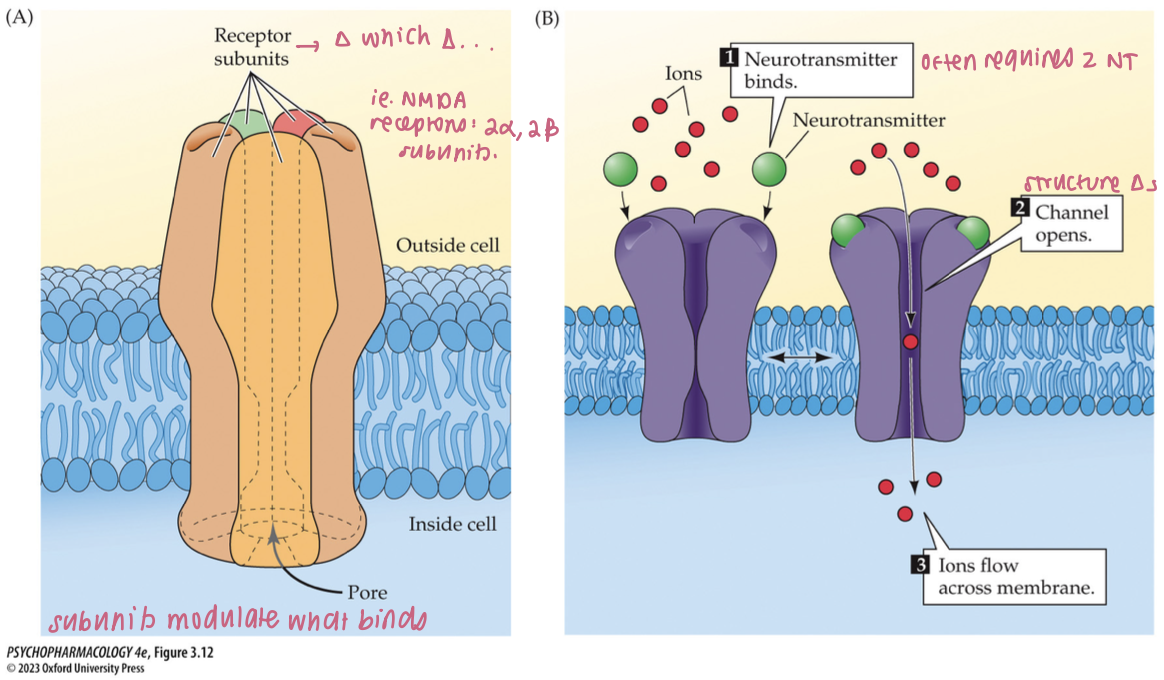
structure + function of ionotropic receptors (4)
consists of multiple subunits that form a selective ion channel for specific ions (K+, Na+, Ca2+, Cl-)
resting state = ion channel closed
NT binding opens the channel → closes when NT dissociates
act rapidly + can undergo desensitization
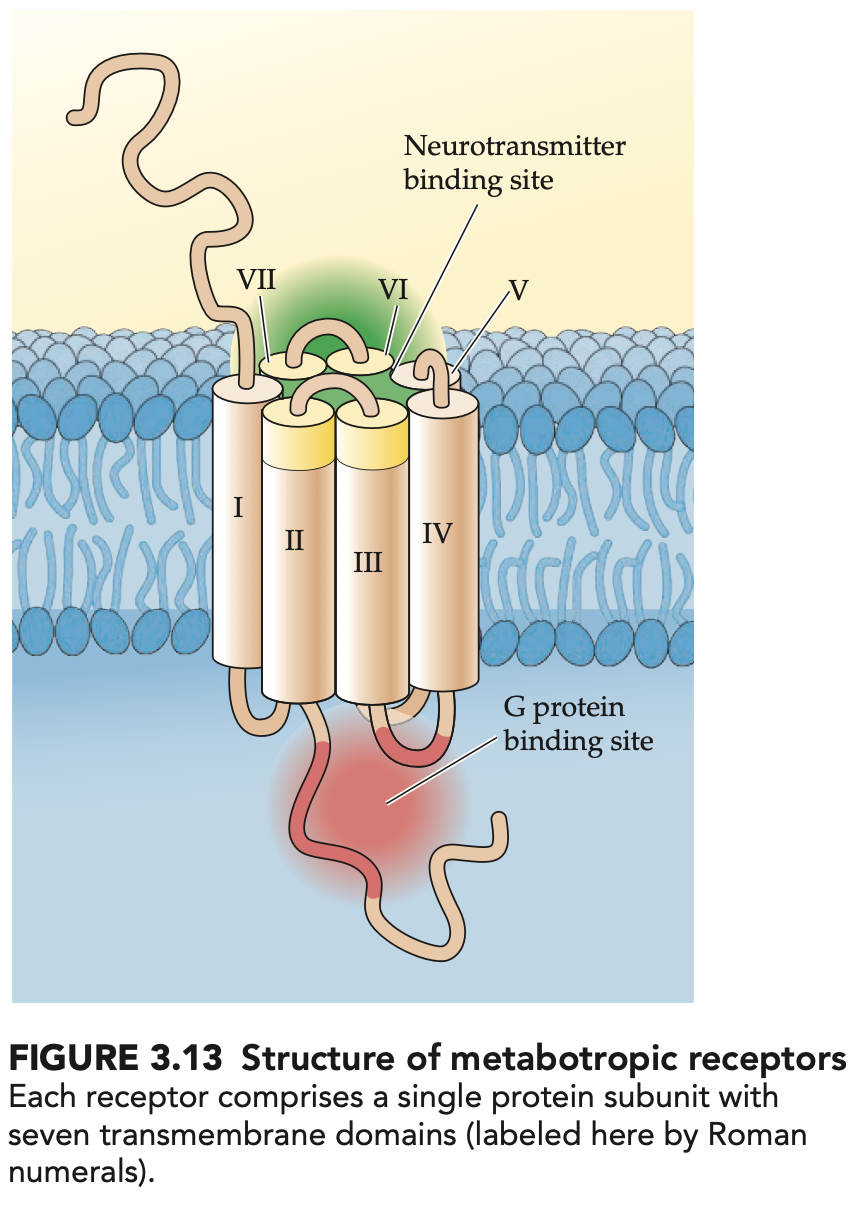
structure + function of metabotropic receptors (4)
consist of a single subunit that works by activating G proteins when NT binds
G proteins open ion channels or stimulate/inhibit membrane effector enzymes
acts slower but response lasts longer
have additional binding sites → allosteric sites
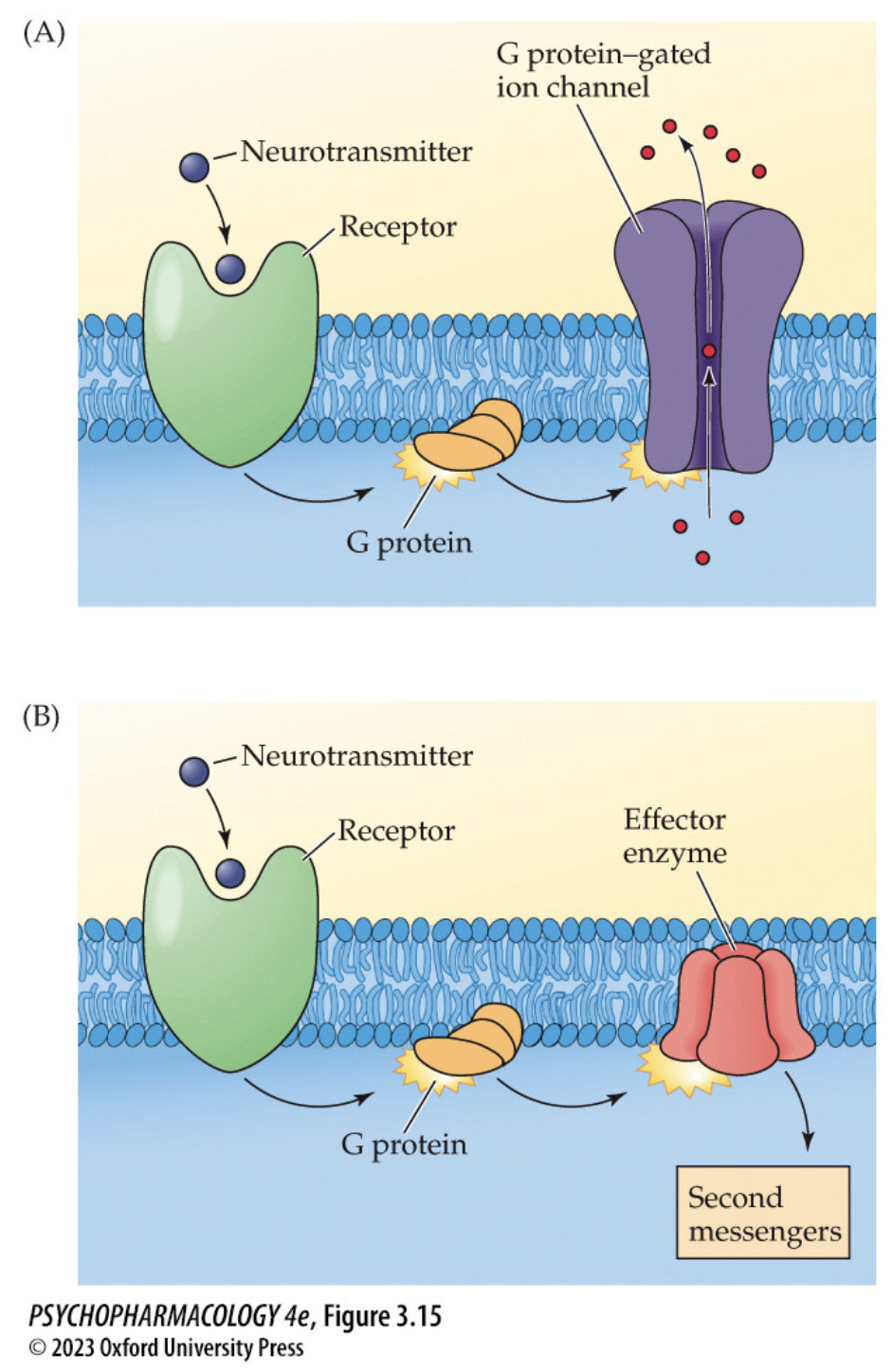
effector enzymes are involved in
synthesis or breakdown of 2nd messengers
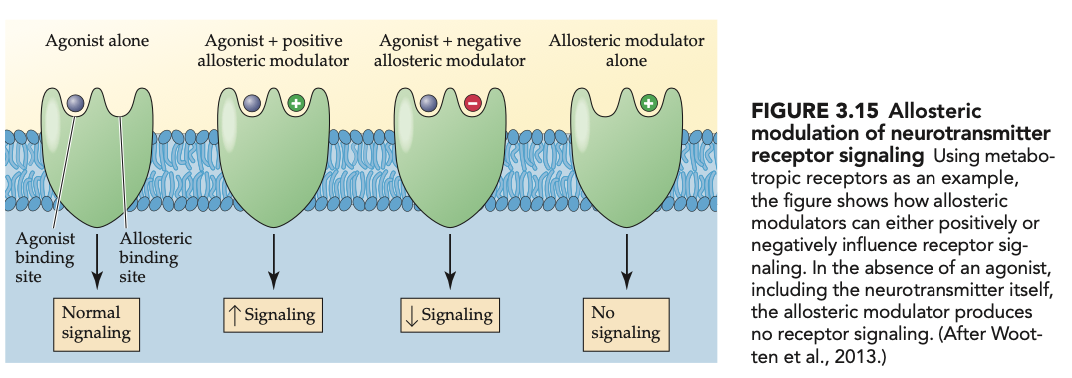
allosteric modulators (2)
bind to allosteric sites + modify (positively or negatively) the effects of an agonist
have potential for treating psychiatric + neurological disorders
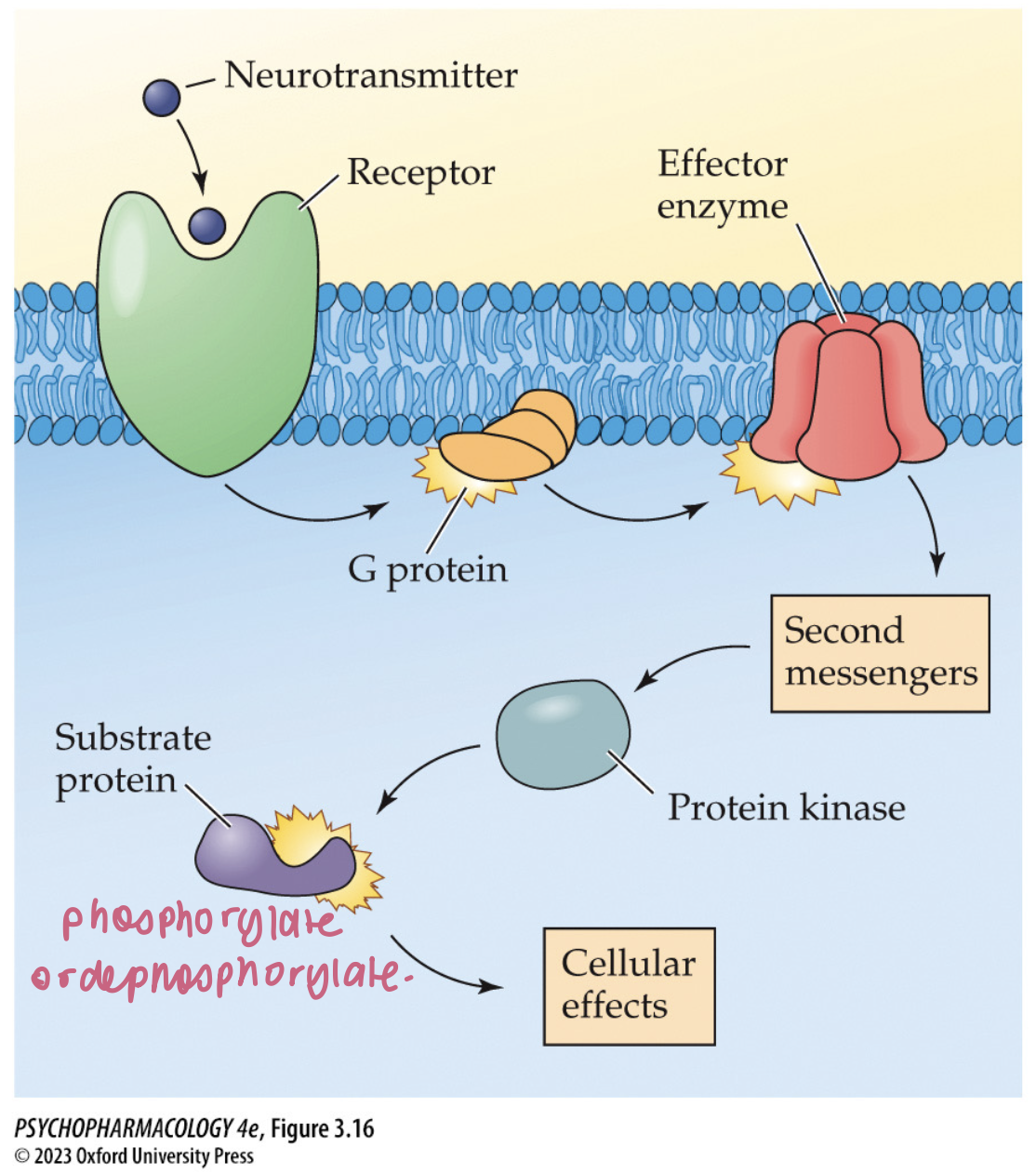
mechanisms of action of 2nd messengers
activate protein kinases that phosphorylate another protein molecule
the added phosphate groups alter functioning of the protein
includes cAMP, gAMP, Ca2+, PIP2
phosphodiesterases (PDEs) + clinical uses (2)
inactivate 2nd messengers like cAMP + gAMP
inhibitors of specific PDEs may be useful in treating various CNS disorders
phosphoinositide second-messenger system (5)
breaks down a phospholipid in the cell membrane to form two 2nd messengers:
diacylglycerol (DAG)
inositol triphosphate (IP3)
they cause ↑sed Ca2+ that activates protein kinase C (PKC)
Ca2+ also activates calcium/calmodulin kinase II (CaMKII)
neurotrophic factors (4)
action mediated by tyrosine kinase receptors
stimulate the survival + growth of neurons during early development
are involved in neuronal signalling
these systems generally participate in regulation of long-term changes in gene expression + neuronal functioning
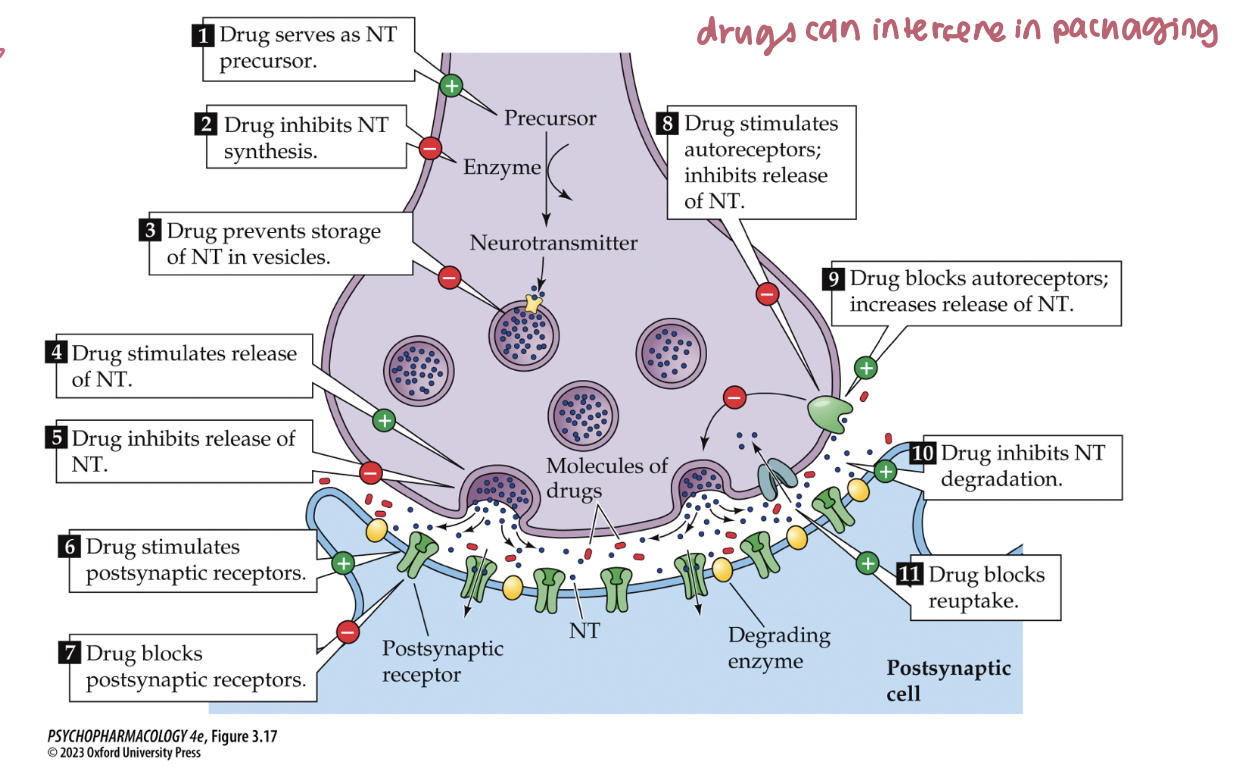
Ways drugs can modify synaptic transmission (overview) (5)
NT Synthesis: ↑ as precursor or ↓ by enzyme inhibition
Storage: block vesicle packaging (e.g., VMAT inhibitors)
Release: stimulate or inhibit exocytosis
Termination: block degradation (AChE/MAO) or block reuptake (SSRIs, cocaine)
Receptors: agonize or antagonize postsynaptic receptors
🧠 Takeaway: Think pre → during → post: make, store, release, stop, receive.
synaptic plasticity (4)
functional ∆s → strength of existing synapses
structural ∆s → loss of synapses/growth of new ones (+dendritic spines)
∆ in dendritic length, branching, spine density
many abused drugs produce ∆s in neuron dendrites
adrenal glands secrete (2)
adrenal medulla secretes epinephrine + norepinephrine (monoamines)
adrenal cortex: secretes glucocorticoids → steroid hormones
gonads secrete (2)
ovaries: estrogen + progesterone
testes: androgens → testosterone
islets of Langerhans in the pancreas secrete (3):
insulin
glucagon
peptide hormones for the regulation of glucose
thyroid gland secretes (3)
thyroxine (T4)
triiodothyronine (T3)
regulate energy metabolism
melatonin is secreted by the ____ and controls ____
pineal gland
sleep + other rhythms
anterior pituitary secretes stimulation hormones (6)
TRH → TSH
CRH → ACTH
GnRH → FSH
GnRH → LH
GH
PRL
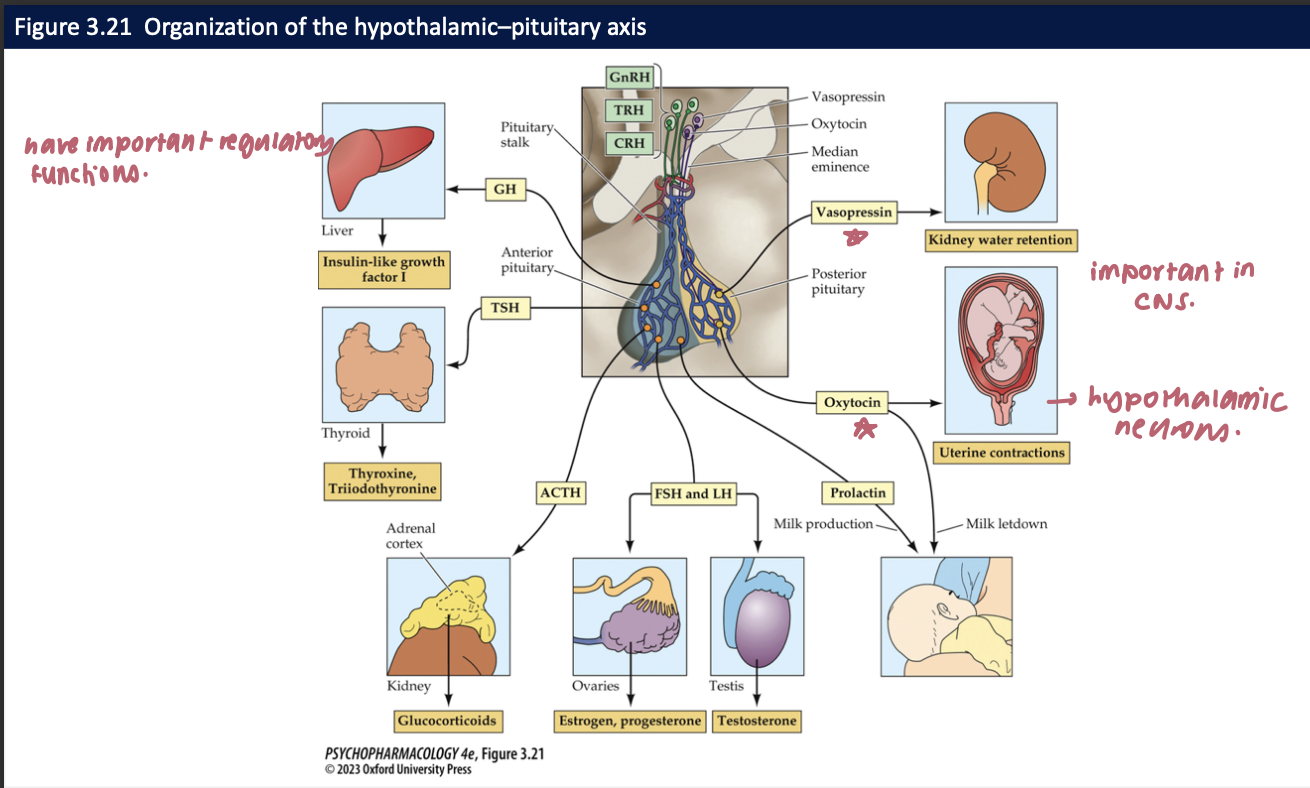
hypothalamus secretes ____ to trigger secretion of _____
releasing hormones
stimulating hormones by the anterior pituitary
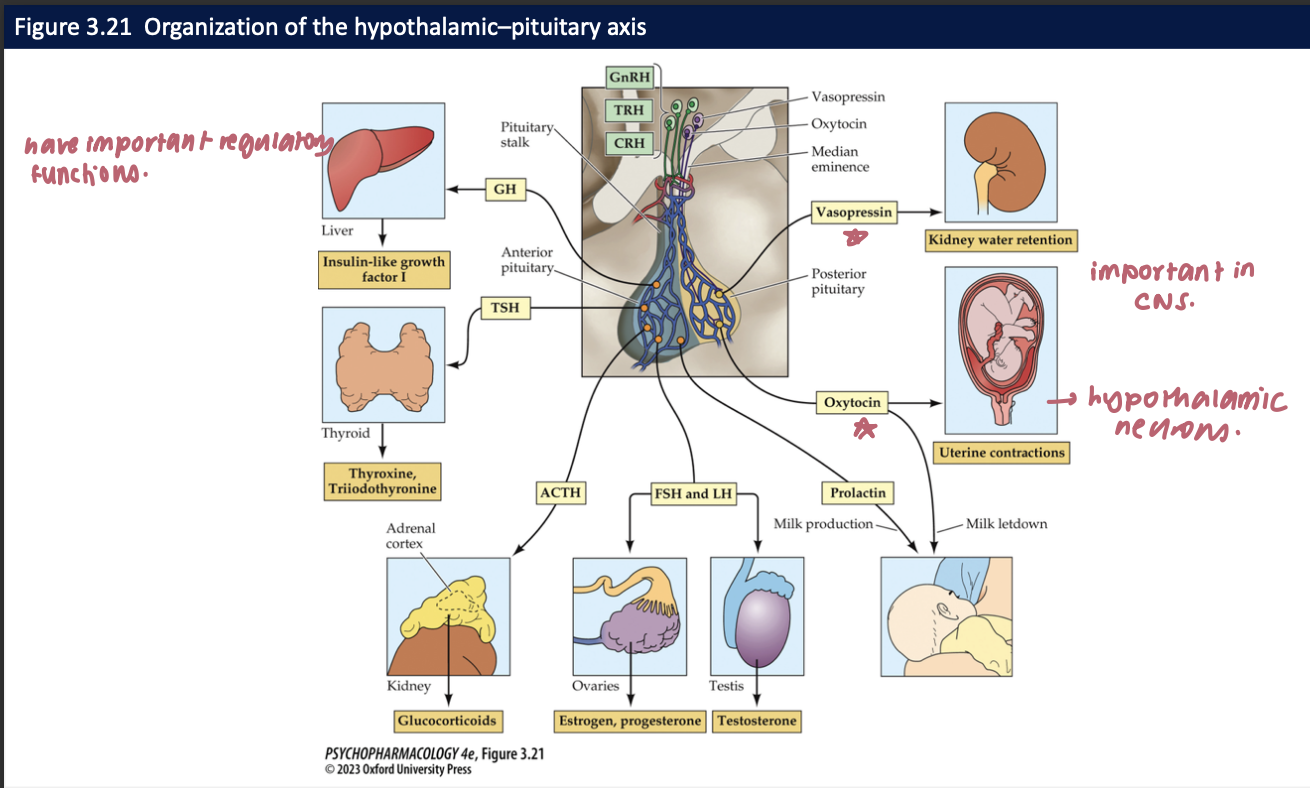
_______ are relased by neurons in the median eminence and are then carried by _____ to the ______
hypothalamic releasing hormones
blood vessels in pituitary stalk
anterior pituitary
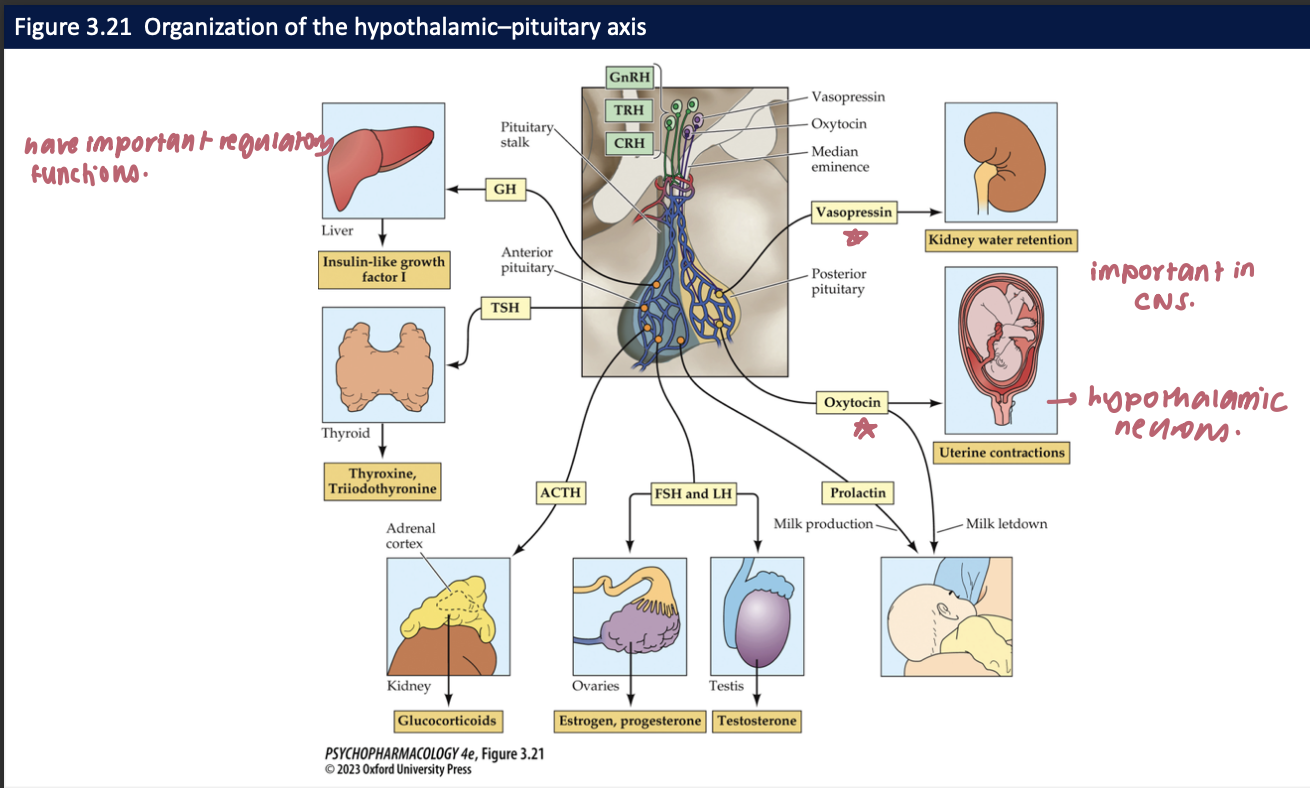
__1__ + __2__ are synthesized in the hypothalamus by neurons whose axons reach the posterior pituitary gland (+ their functions) → they are __3__ + influenced by __4__
vasopressin: regulates water retention in kidneys
oxytocin: stimulates uterine contractions during childbirth → triggers milk letdown from the breasts
sexually dimorphic
gonadal steroids
VP + OT neurons from the _____ connect to many other brain regions which are part of or interact w the _______
paraventricular nucleus
social behavioural neural network
oxytocin can influence a variety of _______, including: ____, altruism, ____, and social memory + it may have potential in what kinds of treatments?
social behaviours
empathy
trust
may have potential in ameliorating the social deficits in autism spectrum disorder patients
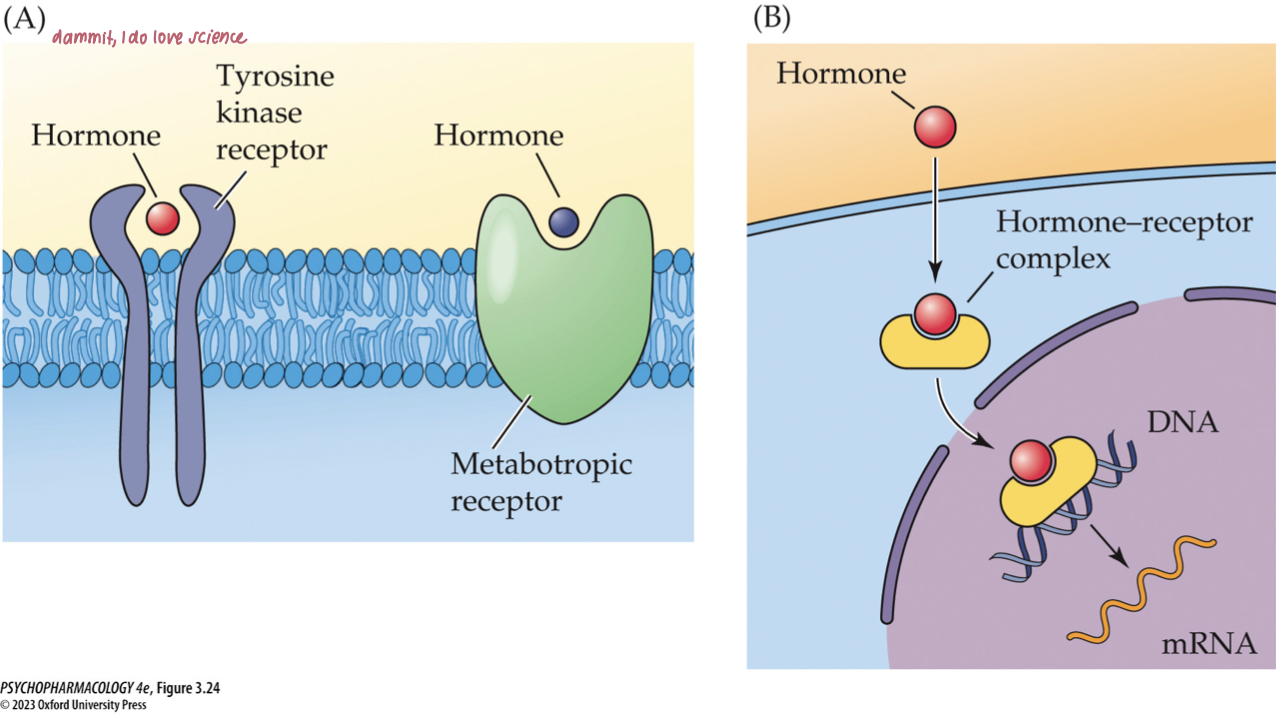
mechanism of hormone action
Most peptide hormones act through membrane metabotropic receptors.
Insulin uses tyrosine kinase receptors.
Steroid and thyroid hormones operate mostly through intracellular receptors in the cell nucleus; function as transcription factors.
why is the endocrine system important to pharmacologists? (4)
Drugs can adversely alter endocrine function.
Hormones may alter behavioural responses to drugs.
Hormones sometimes have psychoactive properties.
Because pituitary hormones are controlled by neurotransmitters in the brain, the endocrine system can tell us if a neurotransmitter system has been altered.